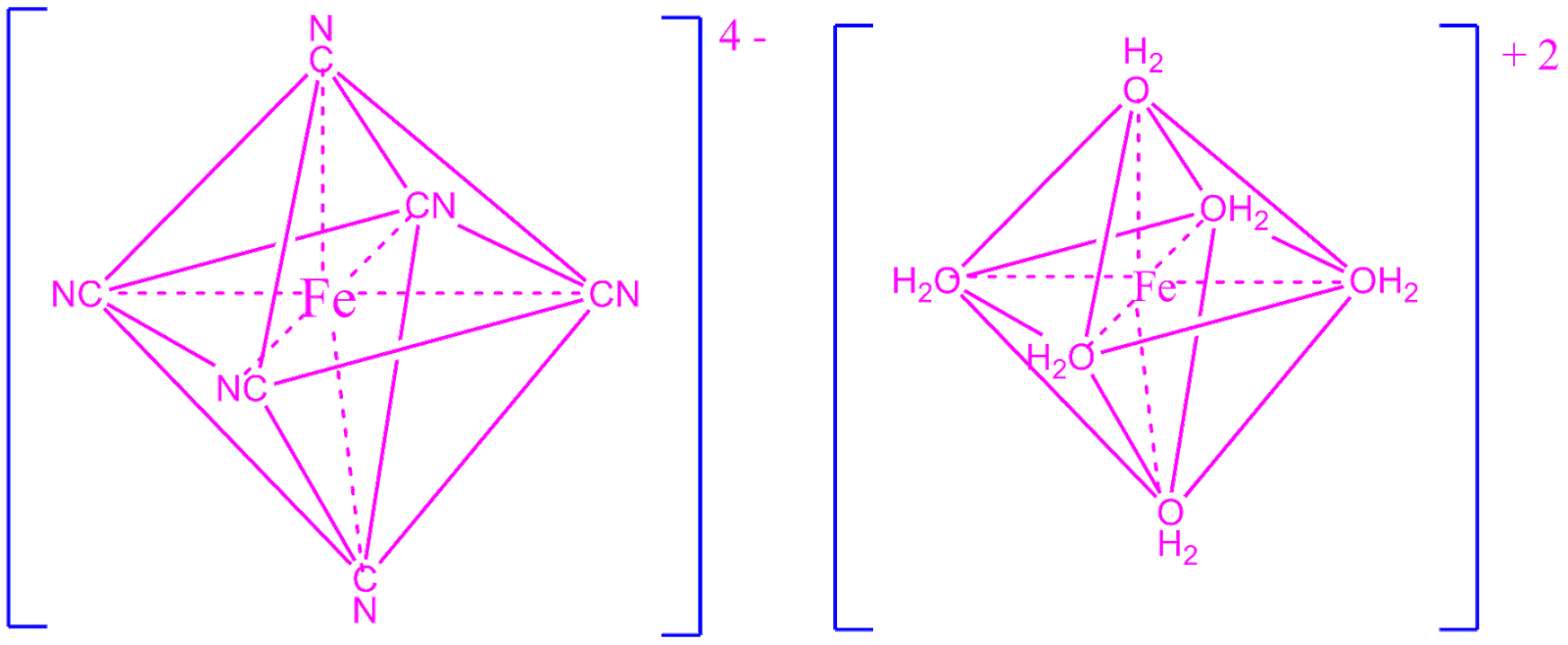
Fecn6 4 Structure

Hybridisation And Shape Of Fe Cn 6 3 Brainly In

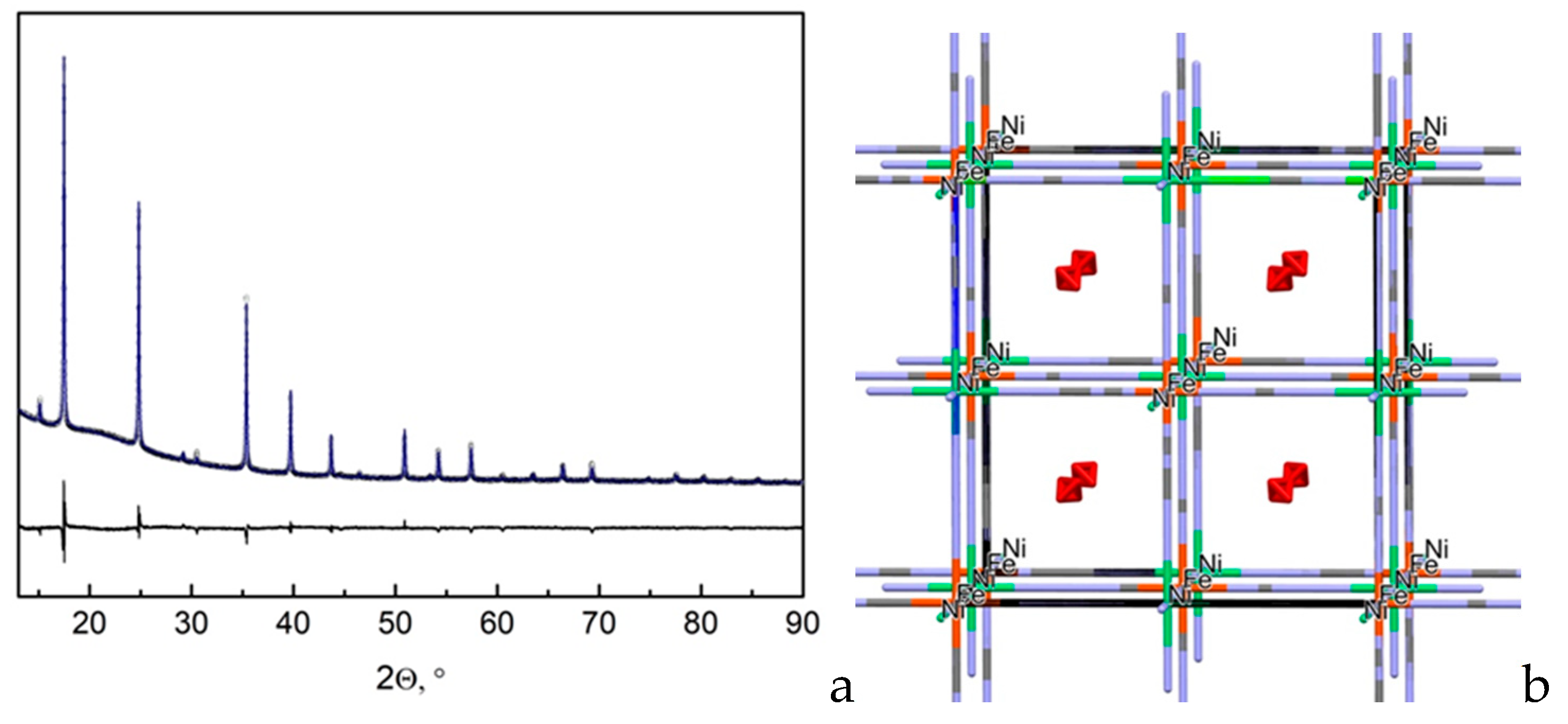
Powder Xrd Pattern Of H Mel 4 Fe Cn 6 Cl A Experimental B Download Scientific Diagram

What Is The Oxidation Number Of Co Cn 6 3 Quora

Name The Following Coordination Entities And Describe Their Structures I Fe Cn 6 4 Ii Cr Nh3 4cl2 Iii Ni Cn 4 2 Atomic Numbers Fe 26 Cr 74 Ni 28
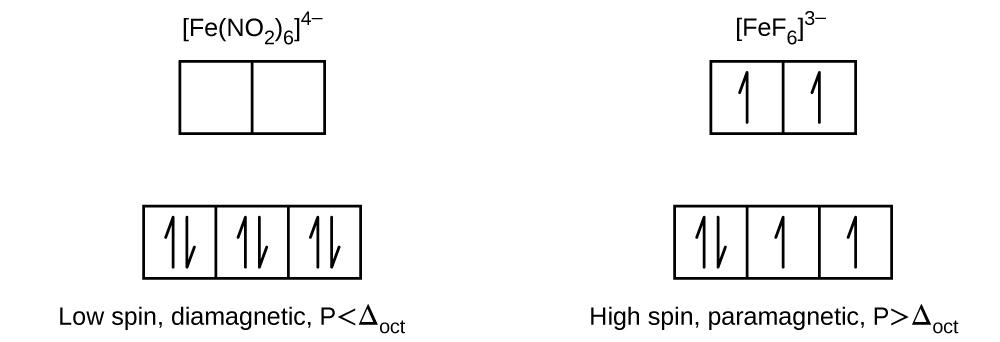
21 3 Spectroscopic And Magnetic Properties Of Coordination Compounds General Chemistry 1 2
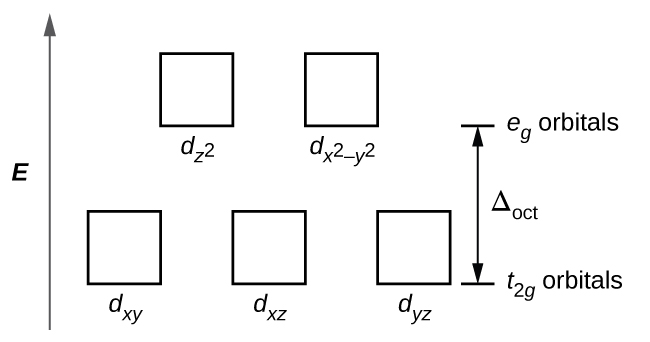
21 3 Spectroscopic And Magnetic Properties Of Coordination Compounds General Chemistry 1 2
Balance the reaction of FeCl2 + K4(Fe(CN)6) = Fe2(Fe(CN)6) + KCl using this chemical equation balancer!.

Fecn6 4 structure. Of electrons in Fe 2+ = 24. It is of commercial interest as a precursor to. Structure of Co ordination Compound Fe CN6 4 on Basis of VBT Video Lecture from Co-Ordination Compounds Chapter of Chemistry Class 12 for HSC, IIT JEE, CBSE.
Citation needed A famous reaction involves treatment with ferric salts to give Prussian blue. For the complex Fe(CN)6^4-, write the hybridization, magnetic character and spin type of the complex. Although many salts of cyanide are highly toxic, ferro- and ferricyanides are less toxic because they tend not to release free cyanide.
Salts of this coordination complex give yellow solutions. Simpler compounds such as the ammonia complex of Co 3+ were known to chemists but did not fit the expected behavior of ionic solids. Free PDF Download of CBSE Chemistry Multiple Choice Questions for Class 12 with Answers Chapter 9 Coordination Compounds.
The tip potential was held constant at ET = 0.5 V to oxidize Fe(CN)6 4-at a diffusion controlled rate. Limitations of Sedgwick Theory •there are number of complexes which are stable but do not follow EAN rule. Chemistry MCQs for Class 12 Chapter Wise with Answers PDF Download was Prepared Based on Latest Exam Pattern.
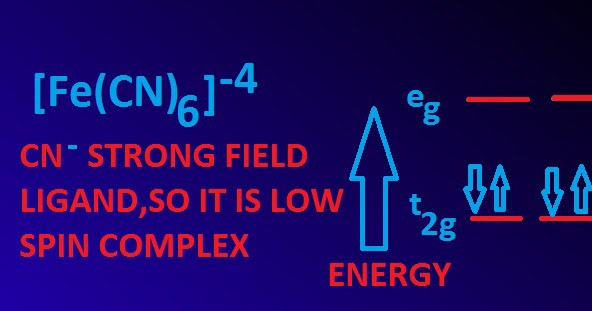
Structure, properties, spectra, suppliers and links for:. Fe(CN)64− is a diamagnetic species, featuring low-spin iron center in an octahedral ligand environment. Tetrapotassium hexacyanoferrate (II) Tetrapotassium hexacyanoferrate (4-) More.
Electronic configuration is 3d6 Orbitals of Fe2+ ion:. 12.5 Fe(H2O)6 3+ and Co(H 2O)6 2+ are high-spin species;. It should be pointed out that today people use hybridization in terms of geometry, not the bonding.
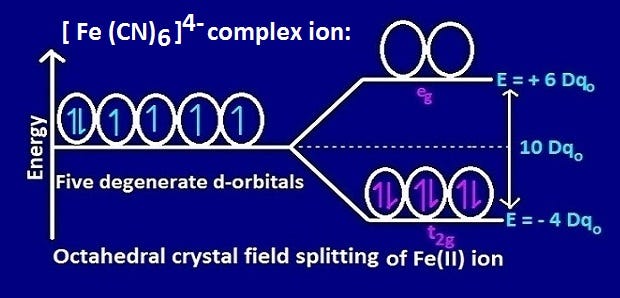
The crystal field splitting of d-electrons of Fe (II) in Fe (CN)64- complex is shown below, Since in this configuration there is no unpaired electron, the complex Fe (CN)64- is diamagnetic. Therefore, the observed magnetic moment is used to determine the number of unpaired electrons present. Computed by PubChem 2.1 (PubChem release ) Topological Polar Surface Area:.
Calculate the value for the overall formation constant of Fe(CN) 6 3-. Students can solve NCERT Class 12 Chemistry Coordination Compounds MCQs Pdf with Answers to know their preparation level. K4Fe(CN)6· 3 H2O or C6FeK4N6+4.
Molecular Weight 859.23. This is fine, if ∆ O is low, as with Fe(H 2 O) 6 2+, and we get a high spin complex, with a higher number of unpaired electrons. The reaction of PPN 2 Mn IV (CN) 6 2− (PPN = N(PPh 3) 2 +) with Fe 11 (NCMe) 4 F 3 CSO 3 2 in MeCN led to the isolation of β-MnFe(CN) 6 exhibiting a new type of powder diffraction pattern for Prussian blue structured materials.The pattern can be indexed to the Fm-3m (or Fm-3) space group with a = 5.55 A ̊ and Z = 8, consistent with two interpenetrating 3-D Prussian blue-type.
(b) Draw one of the geometrical isomers of the complex Pt(en) 2 Cl 2 2+ which is optically active. Coordinated cyanide (Fe(CN) 6 4−) has been introduced into a PANI film by a facile and scalable two-step method:. It is usually available as the salt potassium ferrocyanide, which has the formula K4Fe(CN)6.
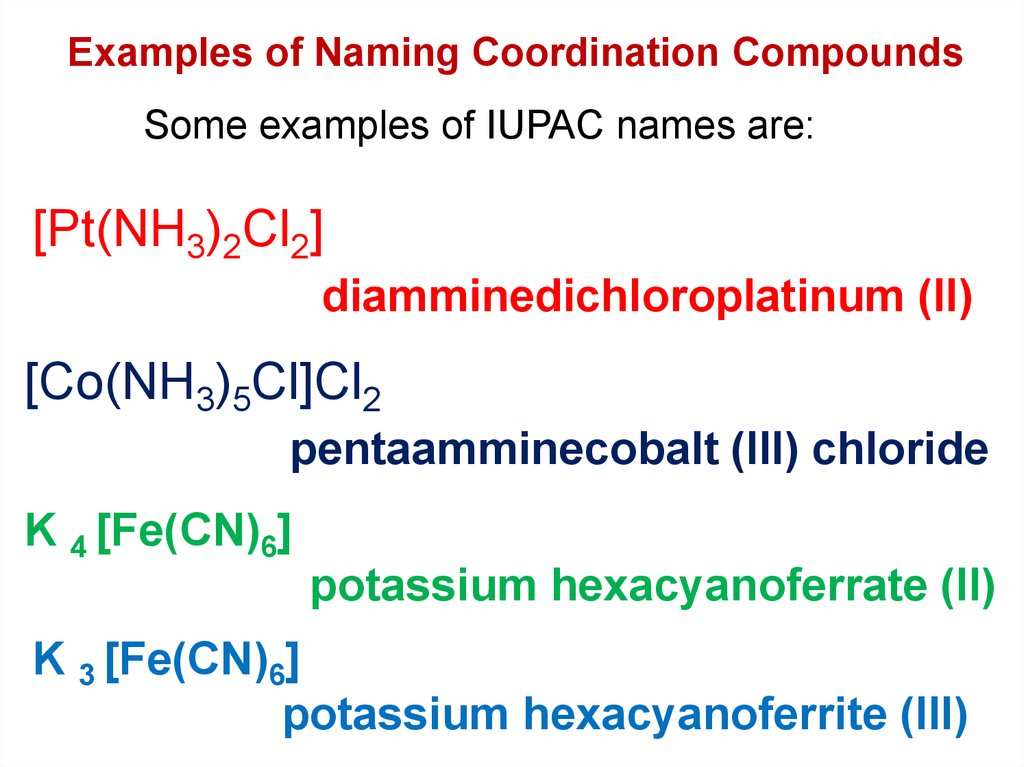
CN-(cyanide ion) named as cyanido The numbers of ligands in a complex are specified using a Greek prefix:. It has a magnetic moment of 6 B.M. Fe(CN) 6 3− + e − ⇌ Fe(CN) 6 4− This redox couple is a standard in electrochemistry.
Ferrocyanide ions, Fe(CN) 6 4– Iron in the +2 oxidation state 4. (a) For the complex Fe(CN) 6 3–, write the hybridization type, magnetic character and spin nature of the complex. (i) Fe(CN) 6 4-In the above coordination complex, iron exists in the +II oxidation state.
Search results for K4Fe(CN)6 at Sigma-Aldrich. Prussian Blue actually contains Fe 3+ cations and Fe(CN) 6 4-anions, and a more descriptive formulation is (Fe 3+) 4 (Fe(CN) 6 4-) 3 •xH 2 O. 0 × 1 0 4 2 _.Given.
In Fe (CN) 6 4−, the strong field of six cyanide ligands produces a large Δ oct. Ferrocyanide is the name of the anion Fe(CN)64−. It is a notably kinetically inert complex, hence its low reactivity towards ligand substitution that would release the potentially toxic cyanide.
The electrons in the upper e g levels render them. Iron can have two oxydation number :. First, verify that the equation contains the same type and number of atoms on both sides of the equation.
The run of reactions with the oxidants (e.g., Fe(III), Fe(CN) 6 3−, Cr 2 O 7 2−, IO 3 −, IO 4 −, BrO 3 −, H 2 O 2, chloramine T) has been studied and employed for determination of used phenothiazine or oxidant.The stability of oxidation products depends on acidity, concentration of oxidizing agents, time, temperature, and the presence of some salts. The six 3 d electrons of the Fe 2+ ion pair in the three t2g orbitals (Figure 3). Electrons from 6CN – ions= 12.
The iron is low spin and easily reduced to the related ferrocyanide ion Fe(CN) 6 4−, which is a ferrous (Fe 2+) derivative. But in Fe (H2O)6+2 the Δ0 value is less than pairing energy resulting in the formation of high spin complex having electronic configuration t2g4 eg2. Hence, the geometry of the complex is octahedral and the complex is diamagnetic (as there are no unpaired electrons).
When Fe(phen) 3 3+ (phen is orthophenanthroline) is reduced by Fe(CN) 6 4-, no ligand bridge forms between the metals and an electron moves from the HOMO of Fe(II) to the LUMO of Fe(III) in a very short and direct contact between the two complexes. Which one of the following complexes is an outer orbital complex?. (i) Fe(CN) 6 4− (ii) FeF 6 3− (iii) Co(C 2O 4)3 3− Ans.
Computed by Cactvs 3.4.6.11 (PubChem release ) Exact Mass:. Fe (CN)6^4- is an octahedral molecule. D 6, with = 4.9 µ B;.
Our team of scientists has experience in all areas. This reaction can be used to remove potassium ferrocyanide from a solution. C) tetrachloro–η 2 –etheneplatinate(II) ion Pt(CH 2 =CH 2)Cl 4 2– has Pt in the +2 oxidation state, which is d 8, and approximately an octahedral crystal field so LFSE = –12Dq.
It is of commercial interest as a precursor to. Fe(III) + e⁻ ⇋ Fe(II) E = 0.36 V. To determine hybridization of Co ordination compound first of all we will have to write electronic configuration of central metal.
Atomic structure (1) Chemical bonding and molecular structure (338) Chemical thermodynamics (359) Solutions (192) Equilibrium (233) Redox reactions and electrochemistry (191) Chemical kinetics (226). (i) Fe(CN) 6 4− In the above coordination complex, iron exists in the +II oxidation state. It is Berlin blue ( Ferric ferrocyanide.
Fe(CN)6^3- Fe - 6CN^- = 3+ This is an Fe(III) cmplx Likewise Fe(CN)6^4- is Fe(II) So half rxns are. Computed by PubChem 2.1 (PubChem release ) Monoisotopic Mass:. F (HFe(H O) 3+ ihihi ith 5 i d l t It h ti t f 2 6 3+ ions are high-spin with 5 unpaired electrons.
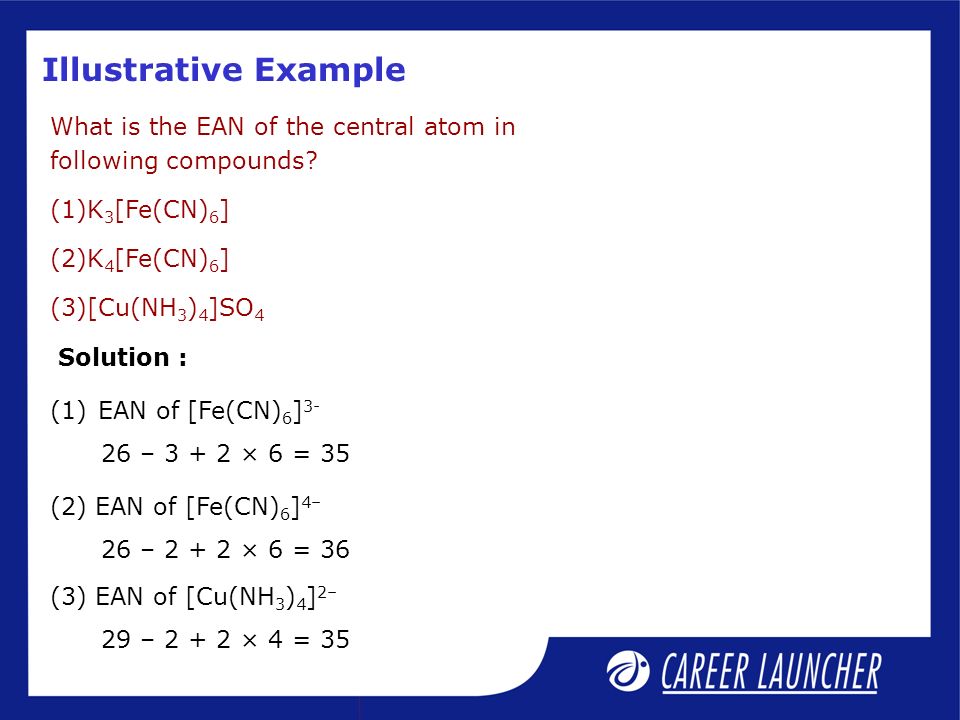
Computed by Cactvs 3.4.6.11 (PubChem release ) Heavy Atom Count. Fe(CN)64− is a diamagnetic species, featuring low-spin iron center in an octahedral ligand environment. EAN of Fe(CN) 6 4-= 36 •this Rule fails in Ni(CN) 4 2-and other Complexes , EAN of Ni(CN) 4 2-=26 + 8 =34.
Although many salts of cyanide are highly toxic, ferro- and ferricyanides are less toxic because they tend not to release free cyanide. PubChem Substance ID. Select up to 4 products.
Fe(cn) 6 4-+ 61ce 4+ + 258oh-→ fe(oh) 3 + 61ce(oh) 3 + 6co 3 2-+ 36h 2 o + 6no 3- Finally , always check to see that the equation is balanced. The overall formation constant of Fe (CN) 6 3 − for the given reaction. As CN − is a strong field ligand, it causes the pairing of the unpaired 3d electrons.
Since both sources of iron are in the +2 state, the. Colorful Iron Complexes continued 2 21 linn ientifi In ll its esere Part A. 6 ligands = hexa → hexacyanido Oxidation state of the central metal atom is shown with a Roman numeral in parantheses at the end of the.
\\ce{2Fe^{2+}(aq) + Fe(CN)6^{4-}(aq) <=> Fe2Fe(CN)6(s) }\ Many metal ions form ferrocyanide precipitates, so potassium ferrocyanide is not a good reagent for separating metal ions. Add 5 drops of 0.1 M potassium ferrocyanide solution to Tube 1. As CN− is a strong field ligand, it causes the pairing of the unpaired 3d electrons.
Jump to main content Jump to site nav. Prior to SECM imaging experiments the tip electrode was. Ferrocyanide is the name of the anion Fe(CN)64−.
The reaction for the formation of Fe (CN) 6 3 − is, Fe 3 + (a q) + 6CN − (a q) ⇌ Fe (CN) 6 3 − (a q) The overall formation constant of Fe (CN) 6 3 − for the given reaction is 1. * If strong ligand is present then pairing of electron occurs, otherwise no. What is the colour of Co(NH 3 ) 6 3+ 3Cl?.
The K +---NCFe linkages break when the solid is dissolved in water. 12.4 The Fe(CN)6 4– ion is a low-spin d6 complex, with a maximum LFSE of –2.4 o. As the result of the electron transfer, Fe(phen) 3 2+ and Fe(CN) 6 3-form.
63–, Fe(CN) 64– and Cu(NH 3) 62+. Orbitals of Fe 2+ ion:. Under these conditions, the electrons require less energy to pair than they require to be excited to the eg orbitals (Δ oct > P).
You can understand that the first iron has the number +three because it needs 3 groups ferrocyanide to make the compound. Structure Search Protocols & Articles. Salts of this coordination complex give yellow solutions.
With the approximate composition KFe 2 (CN) 6, this insoluble but deeply coloured material is the blue of blueprinting. Asked Nov 5, 18 in Chemistry by Tannu ( 53.0k points) coordination compounds. The groups ferrocyanide have the charge -4, given from 6 ions #CN^-# with charge -1 and a atom of iron with charge +2.
Fe(CN) 6 has a magnetic moment of 2.3 B.M., which is a d5 low-spin complex with one unpaired electron. Thereafter orbital diagram is sketched. 2 K 4 Fe(CN) 6 + Cl 2 → 2 K 3 Fe(CN) 6 + 2 KCl.
This redox couple is reversible and entails no making or breaking of Fe–C bonds:. Electronic configuration of Fe 2+ is 4s 0 3d 6. The polymer consists of octahedral Fe(CN) 6 3− centers crosslinked with K + ions that are bound to the CN ligands.
4Fe(CN)6 (NH4)4Fe(CN)6ammo nium ferrocyanideam monium hexacyanofer rate(4-)tetraammoni um hexacyanoferrate. 10.10 Both M(H2O)6 2+ and M(NH 3)6 2+ should show the double-humped curve of Figure 10.12, with larger values for the NH3 compounds. MM K4Fe(CN)6, 50 mM NaClO4, proceeded by rinsing a sample with copious amounts of mM tris-ClO4 (pH 8.6).
2 K 4 Fe(CN) 6 + Cl 2 → 2 K 3 Fe(CN) 6 + 2 KCl Structure. A double salt dissociates into simple ions completely when dissolved in water such as camallite (KCl.MgCl 2.6FI 2 O) while a complex ion such as Fe(CN) 6 4-does not dissociate into Fe 2+. Explain why Fe(H 2 O) 63+ has magnetic moment value of 5.92 BM whereas Fe(CN) 63– has a value of only 1.74 BM.
So in terms of hybridization notation, it is d2sp3 geometry. Cr(Cl) 63–, Cr(CN) 63–, Cr(NH 3) 63+. Arrange following complex ions in increasing order of crystal field splitting energy ( ∆ O) :.
The equilibrium concentration of Fe 3 + is 8.5. Name the complex ion with the forumla Fe(CN) 6 3-Anionic ligands have names ending in 'o'. The size of the R groups changes the structure enough that it is locked into high-spin species at all temperatures.
(ii) FeF 6 3-In this complex, the oxidation state of Fe is +3. It is usually available as the salt potassium ferrocyanide, which has the formula K4Fe(CN)6. It is used more commonly as a confirmatory test.
Like other metal cyanides, solid potassium ferricyanide has a complicated polymeric structure. Electrodeposition followed by solution-soaking.During solution-soaking, the Fe(CN) 6 3− ions react with the PANI and are reduced to Fe(CN) 6 4− on the PANI chains to form a carboncloth-polyaniline-Fe(CN) 6 4− electrode (referred as CC-PANI-FeCN). Ferric hexacyanoferrate (II)).
Fe(CN) 6 4– has Fe in the +2 oxidation state, which is d 6, and a strong octahedral crystal field so LFSE = –24Dq + 2P. Linear Formula Fe 4 Fe(CN) 6 3. For investigating how different 3d ions influence the magnetic relaxation behaviors of 3d–4f systems, six isomorphic tetranuclear 3d–4f compounds of LnFe(CN) 6 (H 2 L)(H 2 O)(DMF) 2 ·5H 2 O (Ln = Dy(1), Tb(2), Ho(3), (H 2 L = 2,6-diylbis(ethan-1-yl-1-ylidene)-di(isonicotinohydrazide))) and LnCo(CN) 6 (H 2 L)(H 2 O)(DMF) 2 ·5H 2 O (Ln = Dy(4), Tb(5), Ho(6)) were obtained from M(CN) 6.
However, if ∆ O is large, as with Fe(CN) 6 4- , the pairing energy is lower than the energy required to get an electron into the high energy orbitals, so the complex is low spin. The equilibrium concentrations of Fe 3+ and Fe(CN) 6 3-are 8.5 X 10 -40 M and 1.5 X 10 -3 M, respectively, in a 0.11-M KCN solution. Fe (CN) 6 4− In the above coordination complex, iron exists in the +II oxidation state.
*Please select more than one item to compare. The oxidation number of each atom can be calculated by subtracting the sum of lone pairs and electrons it gains from bonds from the number of valence electrons. #Valencebondtheory Valence bond Theory in coordination compounds by unil Yadav sir What is valence bond Theory explain with example Valence bond Theory (VBT).
The measured magnetic moment of low-spin d 6 Fe(CN) 6 4− confirms that iron is diamagnetic, whereas high-spin d 6 Fe(H 2 O) 6 2+ has four unpaired electrons with a magnetic moment that confirms this arrangement.
Chemistry And Stomatology Complex Formation In Biological Systems Ppt Download
Coordination Chemistry

Figure 1 From Thermal Decomposition Of Co En 3 Fe Cn 6 2h2o Topotactic Dehydration Process Valence And Spin Exchange Mechanism Elucidation Semantic Scholar
Nptel Ac In Content Storage2 Courses Questions answers coordination Pdf

Ncert Exemplar Class 12 Chemistry Chapter 9 Coordination Compounds Msr Blog
Name The Following Co Ordination Entities And Describe Their Structure Sarthaks Econnect Largest Online Education Community

A Cv Profiles Of The K 4 Fe Cn 6 C Compositea T2 0 4 0 Vatascan Download Scientific Diagram
Nptel Ac In Content Storage2 Courses Questions answers coordination Pdf

Sodium Nitroprusside Wikipedia

Crystal Field Theory Atoms And Electrons
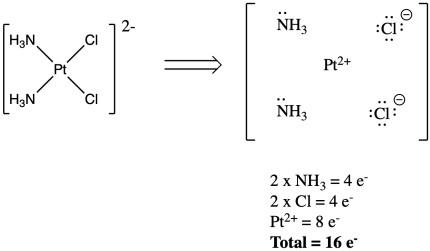
Coordination Chemistry

亚铁氰酸盐 维基百科 自由的百科全书

Chapter 9 Coordination Compounds
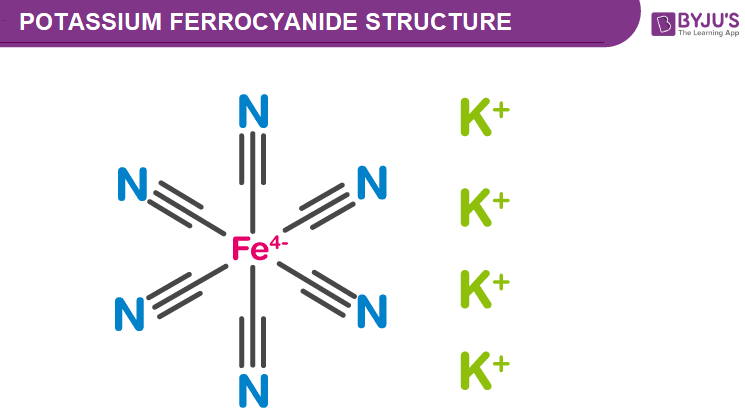
Potassium Ferrocyanide Structure Properties Uses Of K4fe Cn 6
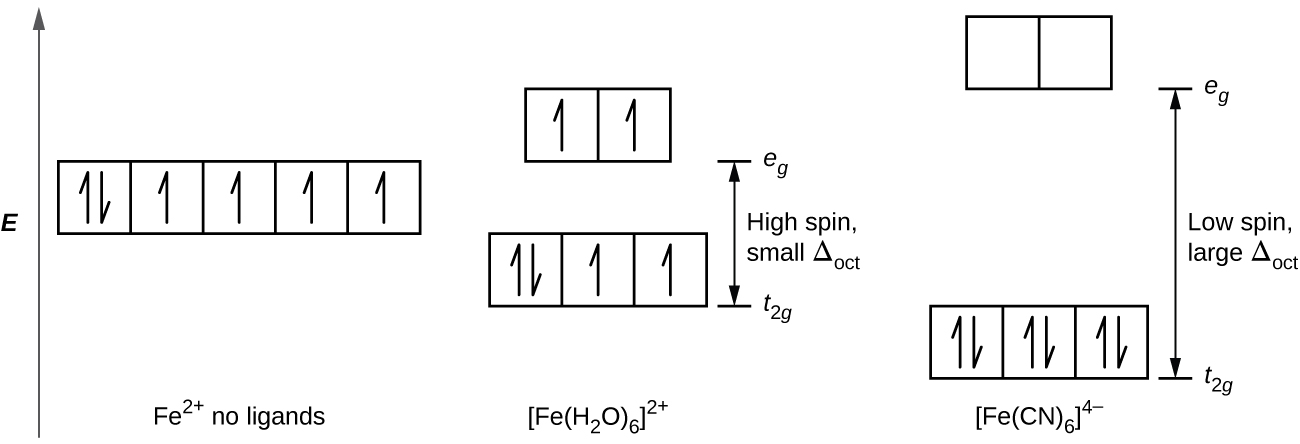
21 3 Spectroscopic And Magnetic Properties Of Coordination Compounds General Chemistry 1 2

22 1 Sodium Hexacyanoferrate Ii Decahydrate Sodium Ferrocyanide Decahydrate Sodium Ferrocyanide Sodium Hexacyanoferrate Na4 Fe Cn 6 Decahydrate Tetrasodium Hexacyanoferrate 4 Decahydrate Yellow Prussiate Of Soda Na Fe Cn 10
Characterization And Utilization Of Prussian Blue And Its Pigments Dalton Transactions Rsc Publishing Doi 10 1039 C6dtb

Formation Of Complex Compounds The Cations Of Transition Metals Have Great Tendency To Form Complexes With Several Molecules Or Ions Called Ligands The Bonds Involved In The Formation Of Complexes Are Coordinate And Hence The Complexes Are Called
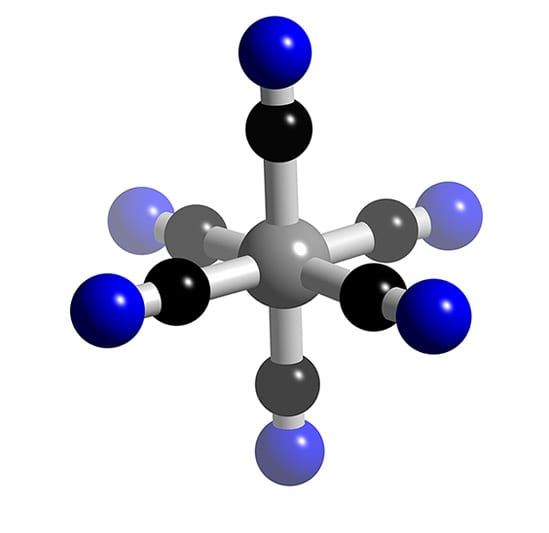
Fe Cn 6 4

Unprecedented Capacity And Stability Of Ammonium Ferrocyanide Catholyte In Ph Neutral Aqueous Redox Flow Batteries Sciencedirect

Potassium Ferrocyanide Wikipedia

Crystal Structure Of Li 3 Fe Cn 6 Viewed Along A Approximately Download Scientific Diagram
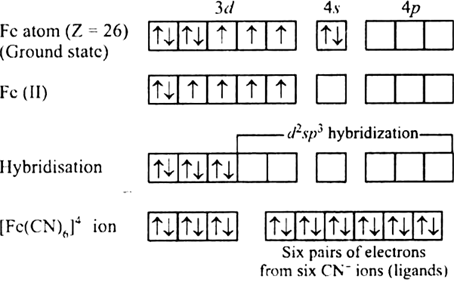
Discuss The Nature Of Bonding In The Following Coordination Entities On The Basis Of Valence Bond Theory Fe Cn 6 4 From Chemistry Coordination Compounds Class 12 Karnataka Board

Sodium Ferrocyanide Wikipedia

For The Complex Ion Fe Cn 6 3 State I The Type Of Hybridisation Ii The Magnetic Behaviour Iii The Oxidation Number Of The Central Metal Atom

Introduction To The Transition Elements Ppt Download

Coordination Chemistry Ppt Download
Q Tbn 3aand9gcr4 S1mjbzvp Hjysma Vv5etnx0nw9zh 8pjqtlfrauqduv To Usqp Cau

Top Representation Of The Molecular Structure Of Net 4 2 Co L R Download Scientific Diagram

What Is The Hybridization Of Cr Cn 6 4 Quora

Ammonium Ferrocyanide Supplier Casno 29 9

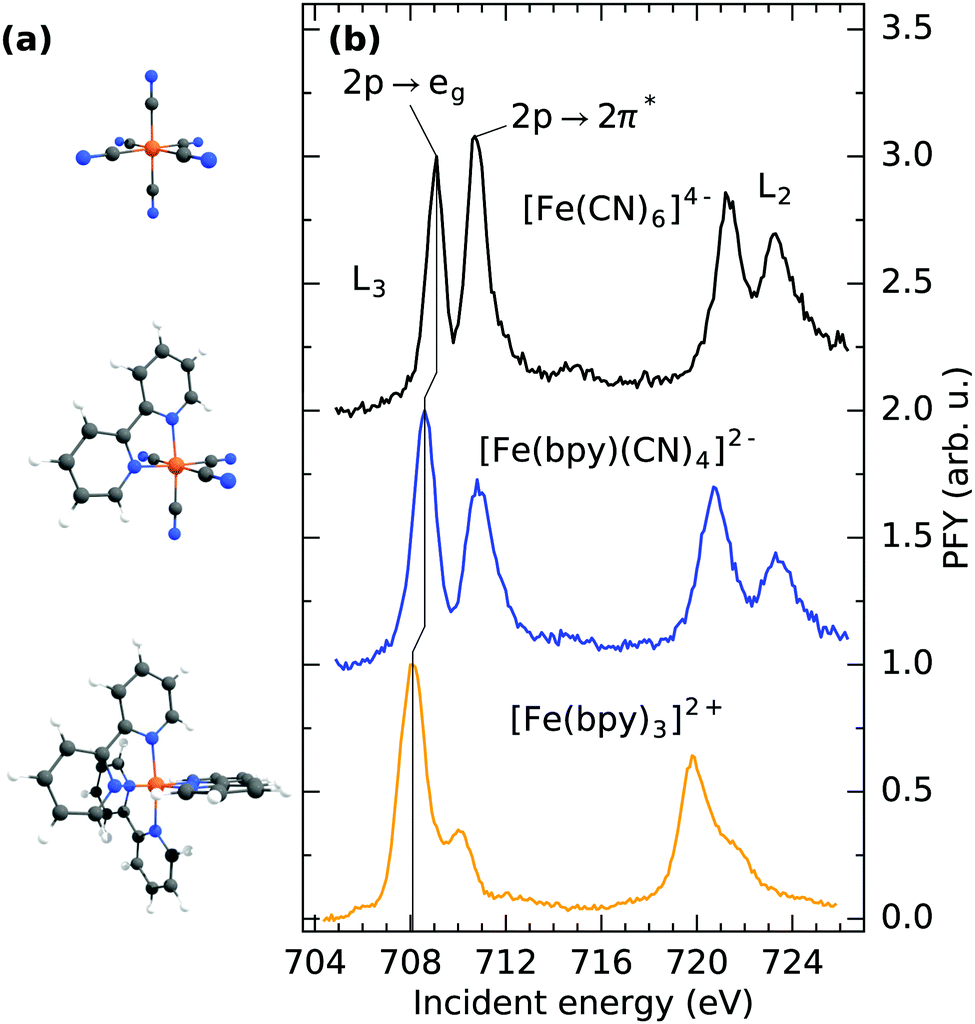
A Molecular Structure And B Fe L 3 2 Edge Partial Fluorescence Download Scientific Diagram

Coordination Compound History Of Coordination Compounds Britannica
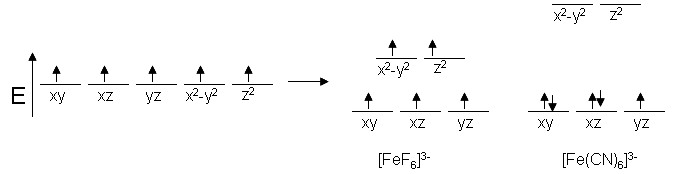
2 11 Magnetic Behavior Of Complex Ions Chemistry Libretexts
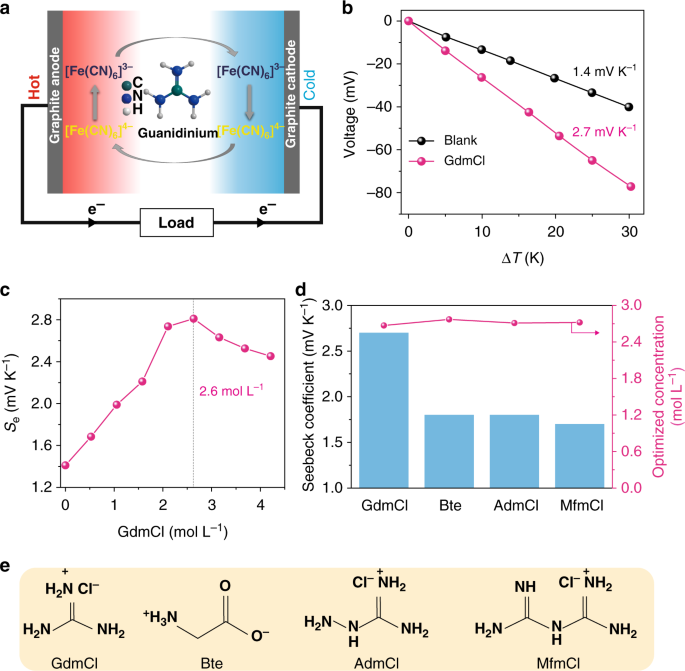
Aqueous Thermogalvanic Cells With A High Seebeck Coefficient For Low Grade Heat Harvest Nature Communications
What Is The Hybridization Of Fe Cn 6 3 Quora

Redox Reactions Of Ru Nh3 6 2 3 Fe Cn 6 3 4 And Fe2 3 On Pristine And Electrochemically Modified Carbon Nanowalls Under Physical Adsorption Of Compounds With The Skeletal And Macrocyclic Structure Sciencedirect
Nanomaterials Free Full Text Formation Of Nanostructured Carbon From Ni Nh3 6 3 Fe Cn 6 2 Html

Figure 4 From Absence Of Quenching By Fe Cn 6 4 Is Not Proof Of Dna Intercalation Semantic Scholar
The Nature Of Frontier Orbitals Under Systematic Ligand Exchange In Pseudo Octahedral Fe Ii Complexes Physical Chemistry Chemical Physics Rsc Publishing Doi 10 1039 C8cph

Figure 1 From High Spin Ni3fe2 Cn 6 And Cu3cr2 Cn 6 Clusters Based On A Trigonal Bipyramidal Geometry Semantic Scholar
Potassium Ferrocyanide C6fek4n6 Chemspider
Chemsolve Net Why Fe Cn 6 4 Is Diamagnetic Where As Fe H2o 6 2 Is Highly Paramagnetic

Draw The Structure Of The Following Compounds A Cis Crcl2 Ox 2 3 B Ni Cn 4 2 C Fe Cn 6 3 D Meridional Crcl3 Nh3 3 E Trans Ptcl2 En 2 2 From Chemistry Coordination Compounds Class 12 Karnataka Board
Chemsolve Net Why Fe Cn 6 4 Is Diamagnetic Where As Fe H2o 6 2 Is Highly Paramagnetic
Nptel Ac In Content Storage2 Courses Questions answers coordination Pdf

Name The Following Coordination Entites And Describe Their Structures Atomic Number Of Fe 26 Cr 24 Ni 28 I Fe Cn 6 4 Ii Cr Nh3 4c12
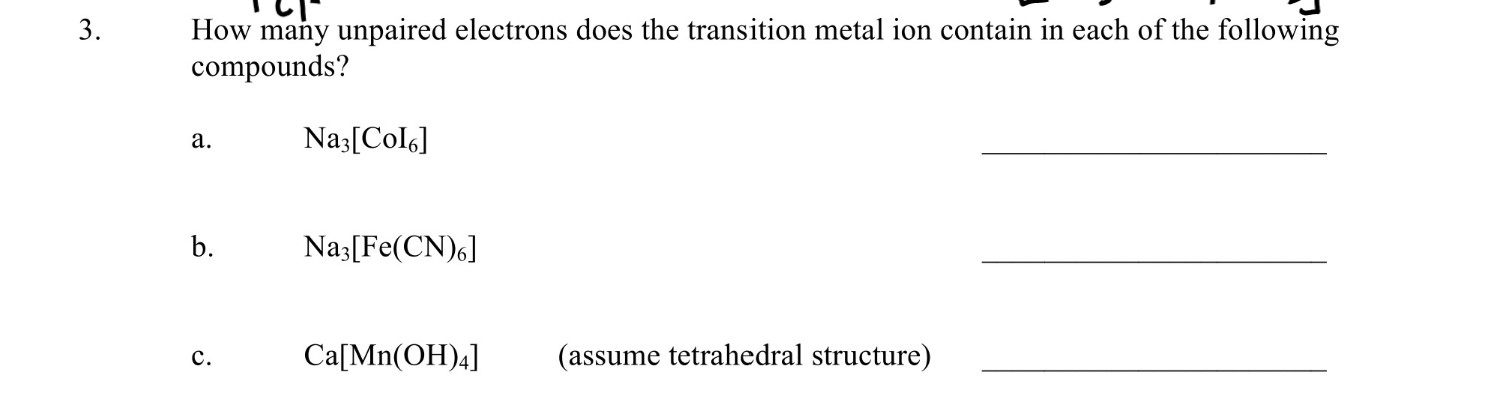
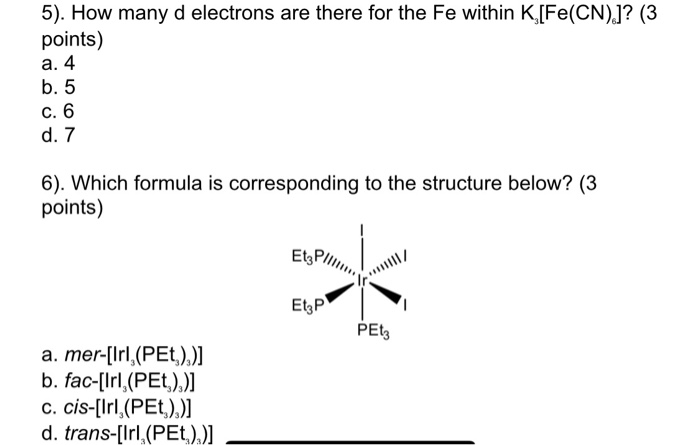
Solved 3 How Many Unpaired Electrons Does The Transition Chegg Com
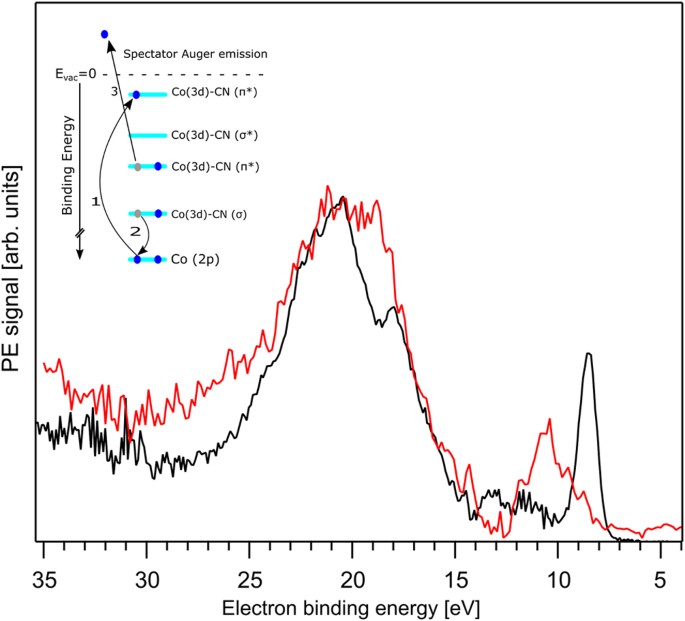
Chemical Bonding In Aqueous Hexacyano Cobaltate From Photon And Electron Detection Perspectives Scientific Reports

Iron Transition Metal Chemistry Iron Ii Fe2 Iron Iii Fe3 Complexes Ions Ligand Substitution Redox Chemical Reactions Principal Oxidation States 2 3 Extraction Gce As Ib A Level Inorganic Chemistry Revision Notes
Http Dx Doi Org 10 Aphyspola 118 696

Schematic Molecular Orbital Diagrams For High Spin 6 A 1g Fe 3 And Download Scientific Diagram
Pubs Acs Org Doi Pdf 10 1021 Acsenergylett 6b

Absence Of Quenching By Fe Cn 6 4 Is Not Proof Of Dna Intercalation Chemical Communications Rsc Publishing Doi 10 1039 C0cce
Tetrapotassium Ferrocyanide C6fek4n6 4 Pubchem

Figure 1 From Crystal Structure And Magnetism Of Pr Fe Cn 6 4 D 2 Semantic Scholar
Www Osti Gov Servlets Purl

The Effective Atomic Number Of Iron In Fe Cn 6 3 Is Youtube
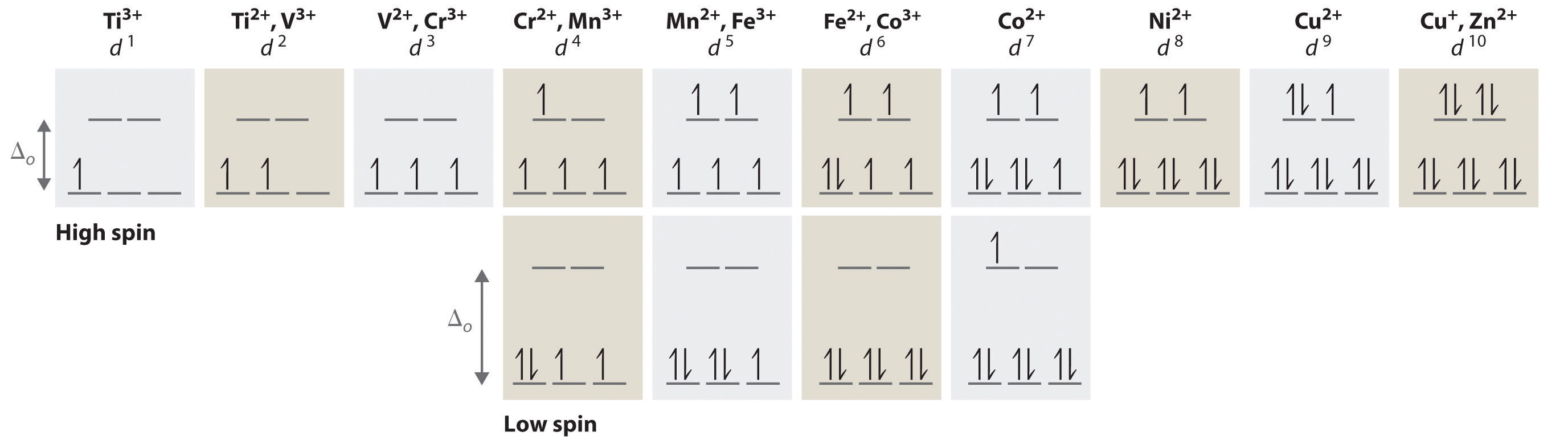
Introduction To Crystal Field Theory Chemistry Libretexts
Ferrocyanide C6fen6 4 Pubchem
Q Tbn 3aand9gcsvuda7bejpgginabnid2vo1l91rcvboh6zvnz9trpnjv0fvesx Usqp Cau
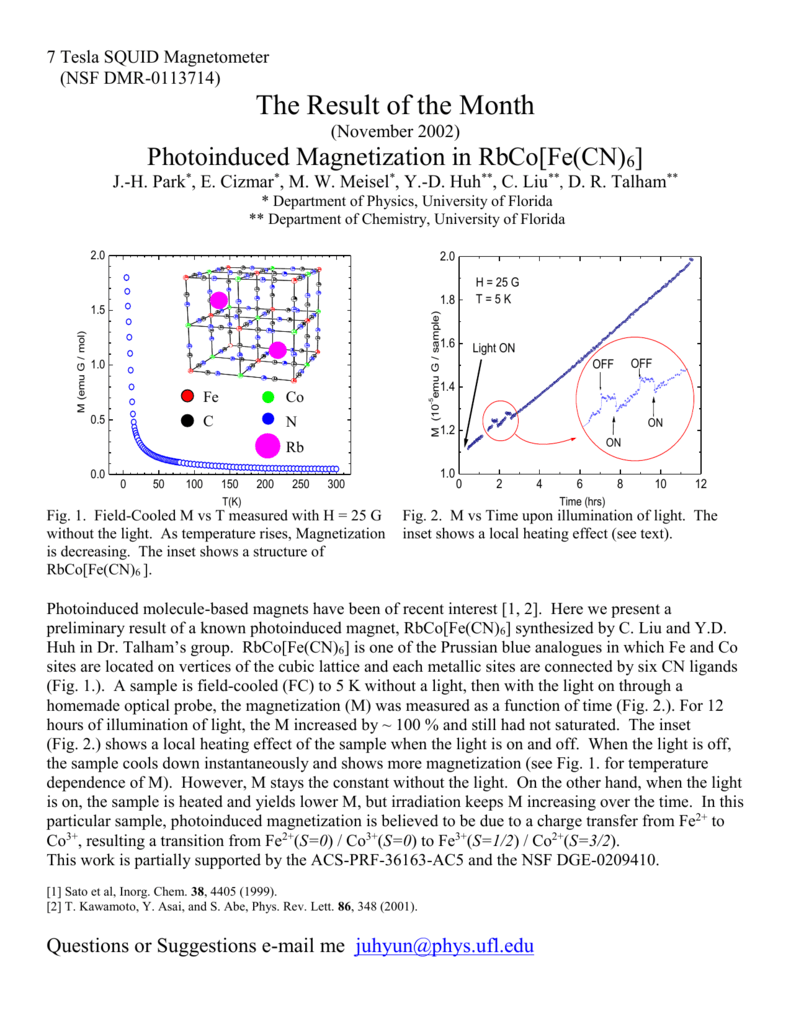
Photoinduced Magnetization In Rbco Fe Cn 6
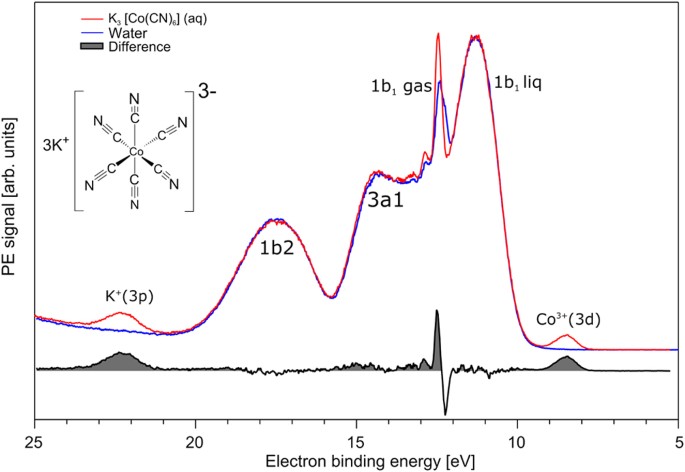
Chemical Bonding In Aqueous Hexacyano Cobaltate From Photon And Electron Detection Perspectives Scientific Reports
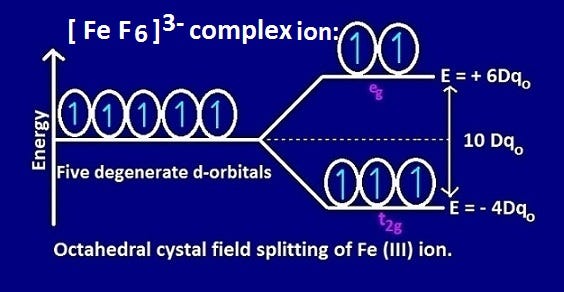
Why Is Fef6 3 Ion Paramagnetic While Fe Cn 6 4 Ion Diamagnetic By Kakali Ghosh Teacher Blogger M Sc Chemistry Medium
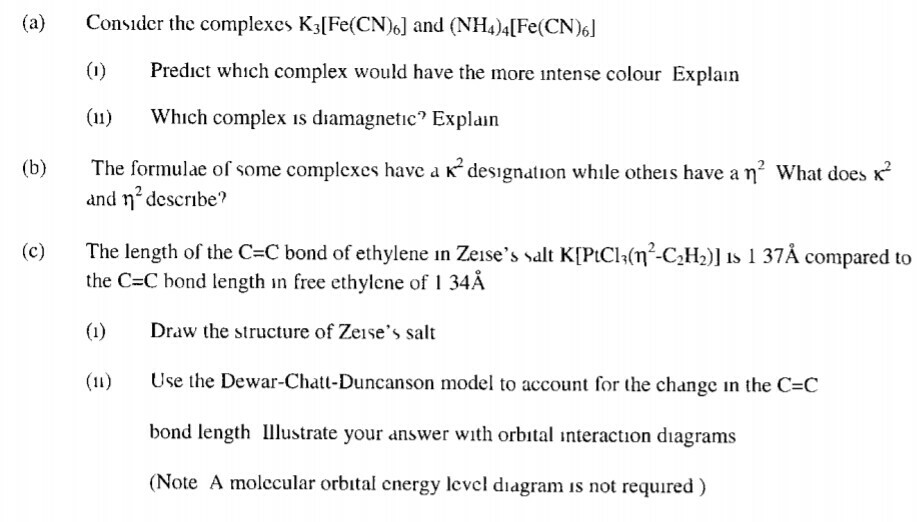
Solved Considcr The Complexes Kalfe Cn 6 And Nh4 4 Fe C Chegg Com

A The Structure Of Co Pba With Composition Co 3 Fe Cn 6 2 The Download Scientific Diagram
Ferricyanide Wikipedia
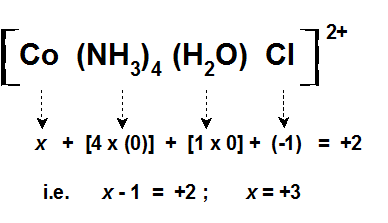
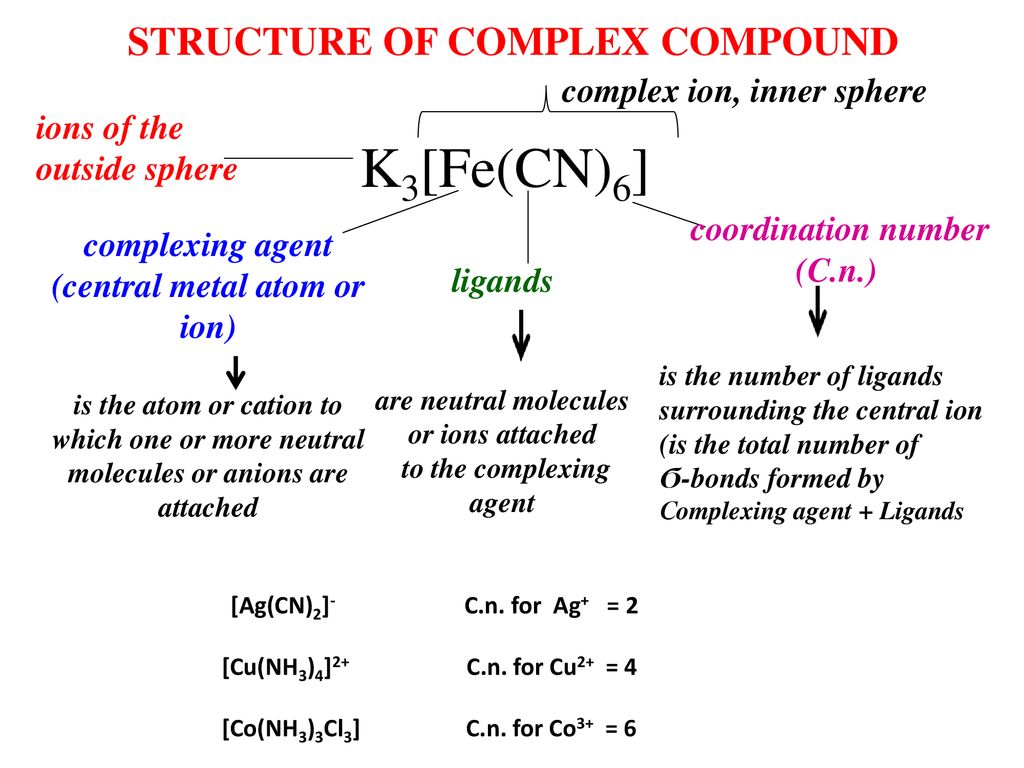
Coordination Compound Ligands Central Metal Atom Or Ion

Schematic Molecular Orbital Diagrams For High Spin 6 A 1g Fe 3 And Download Scientific Diagram
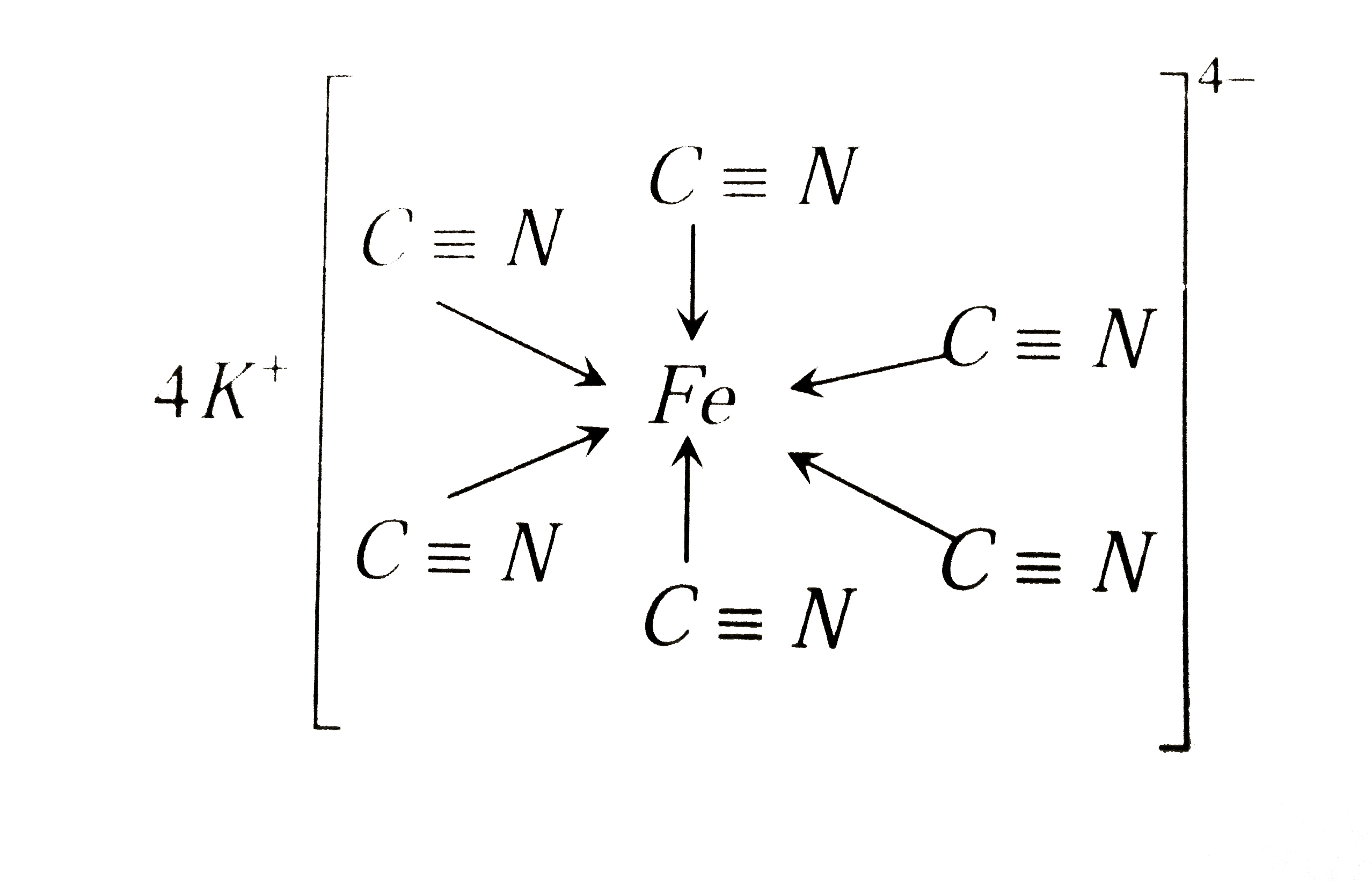
K 4 Fe Cn 6 Is A
Q Tbn 3aand9gct4guu2wef73giuw8usp Brpojjv Gsw4nxyflhgef Xpkrckxd Usqp Cau
What Is The Hybridization Of K3 Fe Cn 6 Quora
Nptel Ac In Content Storage2 Courses Questions answers coordination Pdf
What Is The Hybridization Of The Complex Ion E G Fe Cn 6 4 Quora

Crystal Structure Of Hh Of Nd Dmf 4 H 2 O 3 Fe Cn 6 H 2 O Download Scientific Diagram

Well Defined Na2zn3 Fe Cn 6 2 Nanocrystals As A Low Cost And Cycle Stable Cathode Material For Na Ion Batteries Sciencedirect

Crystal Field Theory Atoms And Electrons

Prussian Blue American Chemical Society
Chemistry Of Coordination Compounds Prezentaciya Onlajn
Nptel Ac In Content Storage2 Courses Questions answers coordination Pdf

Introduction To Inorganic Chemistry Coordination Chemistry And Crystal Field Theory Wikibooks Open Books For An Open World

Synthesis Crystal Structure And Properties Of The Cyano Bridged Heteropolynuclear Cu Meso 3 Co Cn 6 2 9 5h2o Compound

Hybrid Of Fe4 Fe Cn 6 3 Nanocubes And Mos2 Nanosheets On Nitrogen Doped Graphene Realizing Improved Electrochemical Hydrogen Production Sciencedirect
Solved 5 How Many D Electrons Are There For The Fe With Chegg Com

Coordination Chemistry
Why Is Fef6 3 Ion Paramagnetic While Fe Cn 6 4 Ion Diamagnetic By Kakali Ghosh Teacher Blogger M Sc Chemistry Medium
Q Tbn 3aand9gcqfrsz3yhrcuzu7zrqydv9xf5ce F1yroo9tnzxgivkjp6b 74q Usqp Cau
Tetraammonium Hexacyanoferrate 4 C6h16fen10 Chemspider

In Fe4 Fe Cn 6 3 The Oxidation State Of Fe Present In Coordination Sphere Is Brainly In

Table 2 From Crystal Structure And Magnetism Of Pr Fe Cn 6 4 D 2 Semantic Scholar

Coordination Chemistry Ppt Video Online Download

Coordinate Covalent Bond Chemical Bonding Chemistry
Chemistry Ppt Video Online Download

Cyanation Reaction Of Various Aryl Halides Using K 4 Fe Cn 6 In The Download Table
What Is Fe S Oxidation Number In K3 Fe Cn 6 Quora

Structure Of Co Ordination Compound Fe Cn6 4 On Basis Of Vbt Co Ordination Compounds Youtube

K4 Fe Cn 6 Is Supposed To Be 40 Dissociated When 1 M Solution Prepared Its Boiling Point Is Equal Brainly In

A The Structure Of Co Pba With Composition Co 3 Fe Cn 6 2 The Download Scientific Diagram



