Mncn6 4 Structure
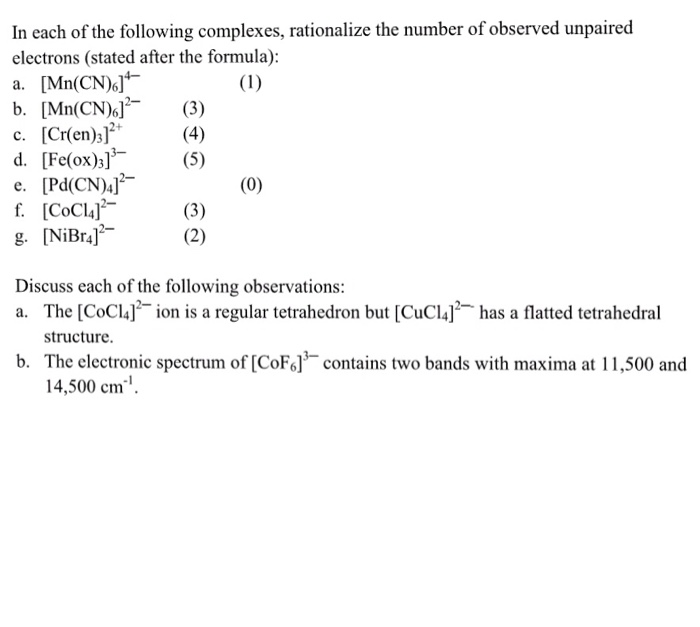
Solved In Each Of The Following Complexes Rationalize Th Chegg Com
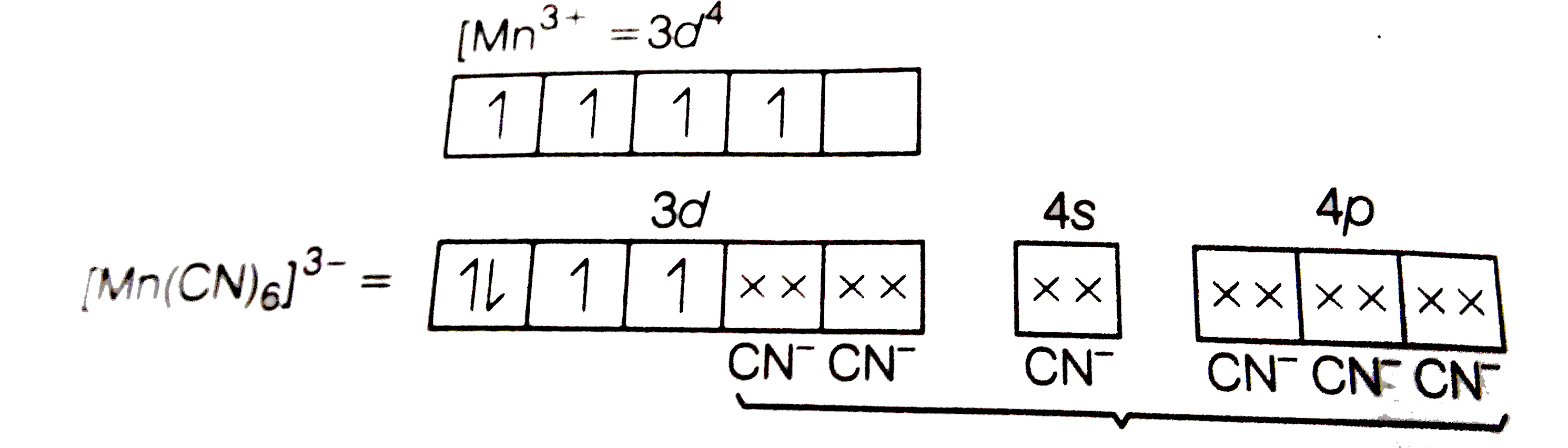
Using Valence Bond Theory Explain The Following In Relation To Th
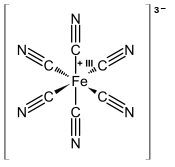
Ferricyanide Wikipedia
Nptel Ac In Content Storage2 Courses Questions answers coordination Pdf
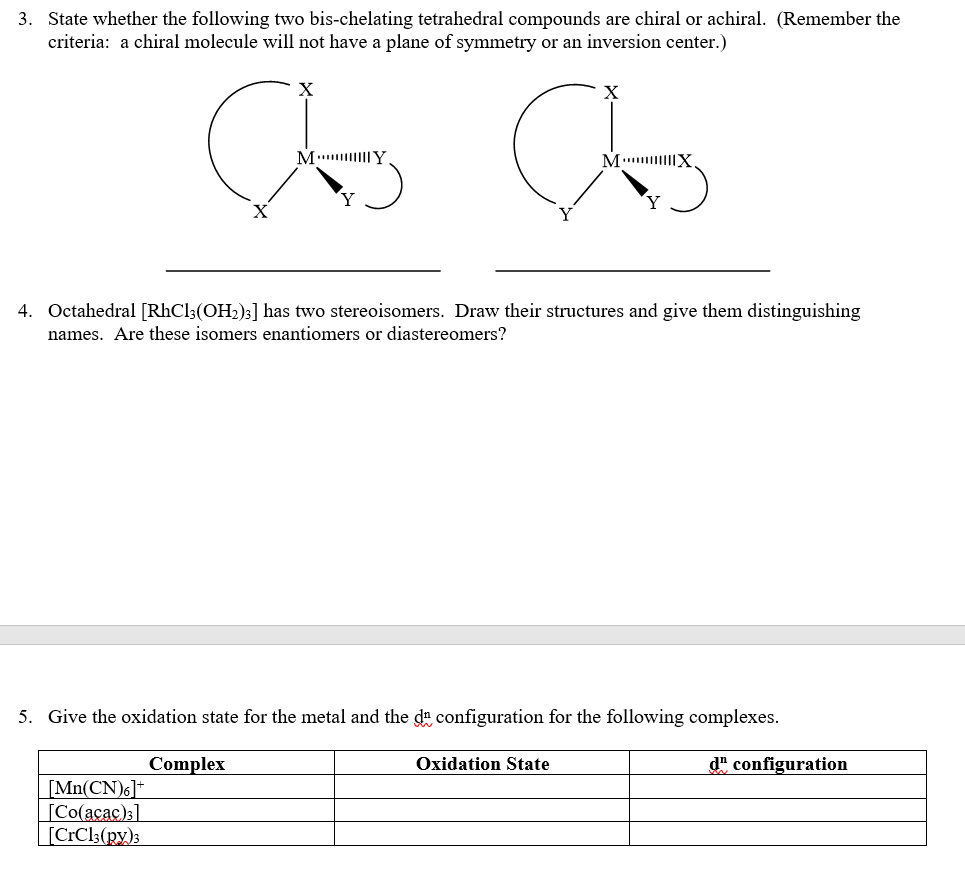
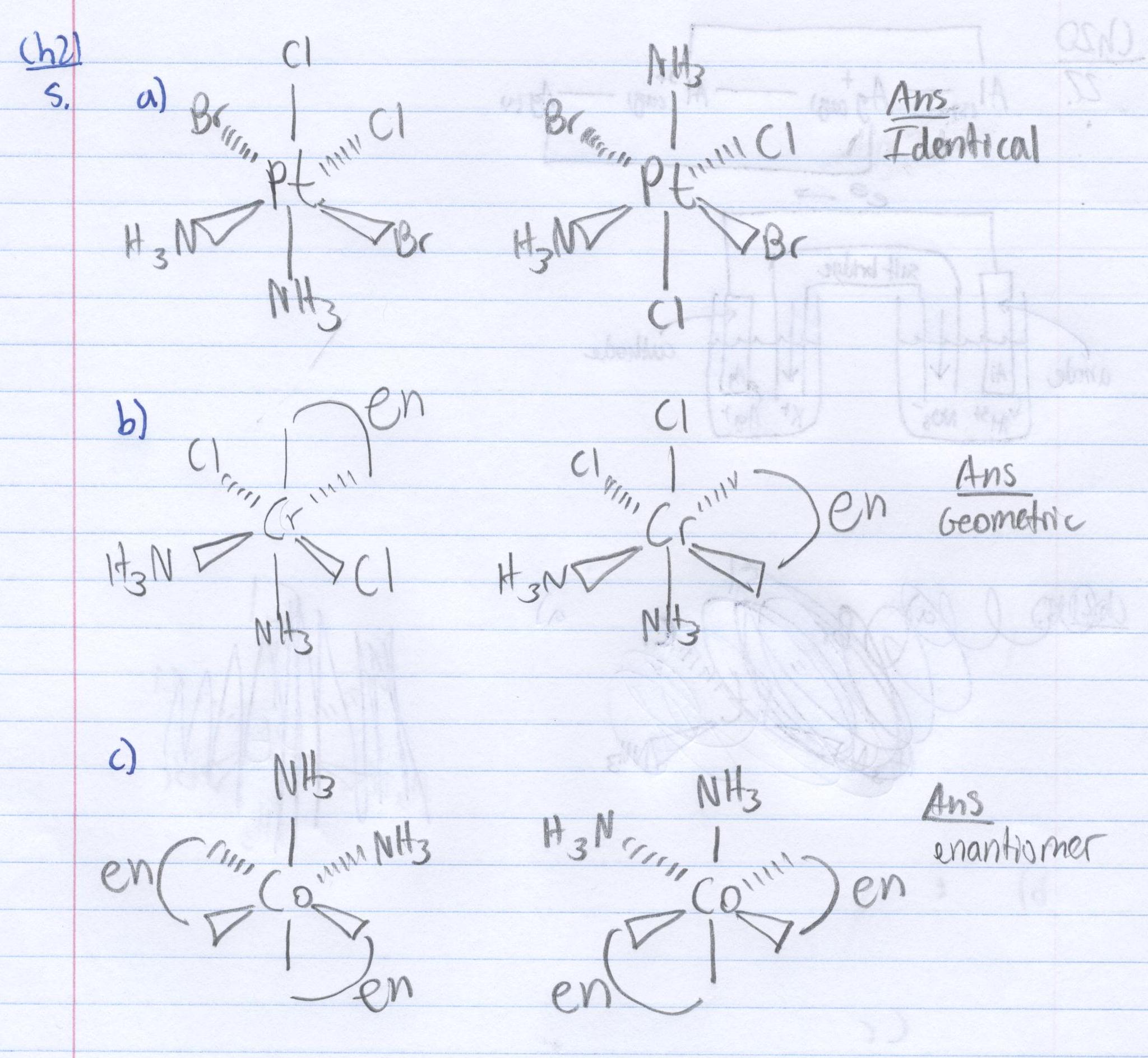
3 State Whether The Following Two Bis Chelating T Chegg Com

Youtube
Introduction to Electronic Structure;.
Mncn6 4 structure. Using the ligand field model, depict the electron configuration for each ion. The oxidation number is synonymous with the oxidation state. Mn is in the +2 oxidation state.
- So, there are 2 vacant 3d orbitals. The anionic nanosized docosanuclear {Mo 8 Mn 14} cluster was the first discrete compound based on Mo(CN) 7 4−. Hitachi participated in the 105th Annual Meeting of the Radiological Society of North America (RSNA), on December 1 -6, 19 at McCormick Place in Chicago, IL.
Therefore, the difference between these curves. • Potassium is the cation, and the complex ion is the anion. Fe(CN) 6 4− is a diamagnetic species, featuring low-spin iron(II) center in an octahedral ligand environment.Although many salts of cyanide are highly toxic, ferro- and ferricyanides are.
The standard heterogeneous rate constant k s of this process in 1 mol dm −3 NaCN is 0.26 ± 0.01 5 cm s −1 and the cathodic transfer coefficient α is 0.59 ± 0.01, similar to the kinetic. Ic7b_si_001.pdf (1.56 MB) Cited By. Ferrocyanide is the name of the anion Fe() 6 4−.Salts of this coordination complex give yellow solutions.
If 4.50 g of the unknown compound contained 0.150 mol of C and 0.300 mol of H, how many moles of oxygen, O, were in the sample?. One of the following nickel complexes ( \(\ce{Ni(H2O)6^2+}\) and \(\ce{Ni(CN)4^2-}\)) is green and the other is yellow. In the compound, Mn is the central atom.
One of the most striking characteristics of transition-metal complexes is the wide range of colors they exhibit (Figure 23.4 "Aqueous Solutions of Vanadium Ions in Oxidation States of +2 to +5" and Figure 23.5 "Compounds of Manganese in Oxidation States +2 to +7").In this section, we describe crystal field theory (CFT) A bonding model based on the assumption that metal–ligand interactions. (iv) Spin only magnetic moment value. The high affinities of metals for this anion can be attributed to its negative charge, compactness, and ability to engage in π-bonding.
6 6-11 Octahedral Ti(III) Complexes Br– Cl– (H2N)2C=O NCS– F– H2O CN– 11,400 13,000 17,550 18,400 18,900 ,100 22,300 Ligand DO/cm–1 • Ti(III) is a d1 complex and exhibits ONE absorption in its electronic spectrum due to transition of the electron from the t2g orbitals to the eg orbitals. Which of the following outer orbital octahedral complexes have same number of unpaired electrons?. The oxidation state of Mn is +3 in this complex.
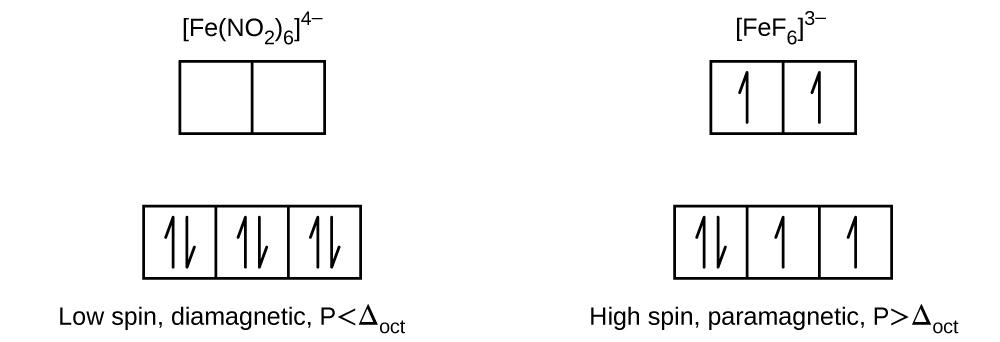
Question_answer7) Fe(H2O)63+ is strongly paramagnetic whereas Fe(CN)63– is weakly paramagnetic. D 6, with = 4.9 µ B;. On the other hand, MnCl63-, FeF63- and CoF63- are outer orbital complexes involving sp3d2 hybridisation and are paramagnetic corresponding to four, five and four unpaired.
(b) Draw one of the geometrical isomers of the complex Pt(en) 2 Cl 2 2+ which is optically active. * In presence of strong field CN-ions, all the electrons are paired up. Like many Mn(II) species, these salts are pink, with the paleness of the color being characteristic of transition metal complexes with high spin d.
The spin only magnetic moments of Mn(CN)64 - & MnBr42 - in bhor magnetons, respectively,are (1.73 and 5.92). The potassium ion is the cation and the complex ion is the anion. The origin of the color in this species is not due to d-d transition, rather, charge transfer from O 2-to Mn(VII), described as LMCT band.
By Bagus Amin - 11:44 AM - Share this. In case of Fe(CN)64− and Fe(H2O)62+, the colour differs because there is a difference in the CFSE. The magnetic moment (μ) of M n is 1.
The electronic configuration is d5. Explain why each metal complex has a different color. For example, Prussian Blue, which has an empirical formula Fe 7 (CN) 18 •xH 2 O, is an insoluble, deep blue solid that has been used as a pigment since its accidental discovery by Diesbach in 1704.
In this complex the charge transfer. The outer electronic configuration is 4s03d44p0. Coordination compounds have been known for centuries, but their structures were initially not understood.
The empty 4d, 3s and two 4p orbitals undergo dsp 2 hybridization to make bonds with CN-ligands in square planar geometry. CCDC (I) and (II) pdf. The trimeric units are connected to each other via hydrogen bonding between CN- and the water of coordination to give anionic chains along the crystallographic a axis.
Prussian Blue actually contains Fe 3+ cations and Fe(CN) 6 4-anions, and a more. Actually its correct structure is not possible(according to EAN rule) so for correct structure their will be two Mn and each Mn will be linked be three CO by. CN- ( Cyanide ) is a Diamagnetic.
The crystal field is octahedral. Tetracyanides M(CN) 4 2− (M = Ni, Pd, Pt), which are square planar in their. The hybridization of Mn(CN)₆³⁻ is d²sp³.
If trimethylphosphine is added to a solution of nickel(II) chloride in acetone, a blue compound that has a molecular mass of approximately 270 g and contains 21.5% Ni, 26.0% Cl, and 52.5% P(CH. (μ) = n (n + 2) = 1 (1 + 2) = 1. 4 answers Is consuming too much alkaline dangerous?.
(CN) 6 3− + e − ⇌ Fe(CN) 6 4. The electronic configuration is d5. Mn (CN)6 has -4 charge CN = -1, so (CN)6 = -6 Mn has +2 charge, which means the Mn ion has 2 less electrons than the Mn atom.
The structure of two manganese hexacyanometallates(II):. * In Ni(CN) 4 2-, there is Ni 2+ ion for which the electronic configuration in the valence shell is 3d 8 4s 0. CoSO 4 Cl.5NH 3 exists in two isomeric forms ‘A’ and ‘B’.
Mn has an oxidation state of +3 so now EC of Mn become 4s^03d^44p^0 CN is a strong field ligand and out of 4 electrons in 3d 2 of them wil pair and hybridization of compound is d2sp3. 63+ (ii) Mn(CN) 63– (iii) Fe(CN) 64– (iv) Fe(CN) 63– 16. Structure, properties, spectra, suppliers and links for:.
This event featured more than 700 companies which gave RSNA’s almost 53,000 attendees the opportunity to view some of the latest innovations in healthcare. Indicate the color of each, and explain how you came to this conclusion. • Since there are 4 K+ associated with the complex ion (each K + having a +1 charge), the charge on the complex ion must be -4.
Now, CN− is a strong field ligand having a higher CFSE value as compared to the CFSE value of water. Dear Students, Please find the solution attached below. Thus, the oxidation number of Mn in the compound is +1.
Computed by Cactvs 3.4.6.11 (PubChem release ) Heavy Atom Count. Manganese(II) chloride is the dichloride salt of manganese, MnCl 2.This inorganic chemical exists in the anhydrous form, as well as the dihydrate (MnCl 2 ·2H 2 O) and tetrahydrate (MnCl 2 ·4H 2 O), with the tetrahydrate being the most common form. - The outer electronic configuration of Mn³⁺ = 3d⁶, 4s⁰, 4p⁰ - As CN⁻ is a strong field compound, it will pair up the electrons in 3d orbital.
Free PDF Download of CBSE Chemistry Multiple Choice Questions for Class 12 with Answers Chapter 9 Coordination Compounds. Electronic Structure and the Periodic Table;. The structure of 2 comprises trimeric units {Mn(L)(H2O)2Nb6Cl12(CN)6}2- in which each cluster is trans-coordinated by two Mn(L)(H2O)+ cations via the CN- ligand.
7 3 B M. • Since each ligand carries –1 charge, the oxidation number of Fe must be +2. 10.10 Both M(H2O)6 2+ and M(NH 3)6 2+ should show the double-humped curve of Figure 10.12, with larger values for the NH3 compounds.
The prospects of Mn III (CN) 6 3. The complex Mn(H 2 O) 6 2-has five unpaired electrons, whereas Mn(CN) 6 4-has only one. Thus, for the compound to be neutrally charged, Mn must have a charge of +1.
1 -0.6 t 6 -0.6 t 2 -1.2 t 7 -1.2 t 3 -0.8 t 8 -0.8 t 4 -04 0.4 t 9 -04 0.4 t 5 zero 10 zero (ii) Square Planar Complexes d-Orbital Splitting in Square Planar Coordination. What can you conclude about the effects of the different ligands on the magnitude of Δ 0 ?. Mn(H 2O) 6 2+ Mn(CN) 6 4-Mn is in the +2 oxidation state.
As there are 4 K + binding with a complex ion, the charge on the complex ion must be - 4. C) tetrachloro–η 2 –etheneplatinate(II) ion Pt(CH 2 =CH 2 )Cl 4 2– has Pt in the +2 oxidation state, which is d 8 , and approximately an octahedral crystal field so LFSE = –12Dq. 7 3 B M.In this complex N i has d 5 orbitals and has 1 unpaired electrons.
Students can solve NCERT Class 12 Chemistry Coordination Compounds MCQs Pdf with Answers to know their preparation level. In the given compound, is a strong-field ligand. Using the ligand field model, depict the electron configuration for each ion.
Computed by PubChem 2.1 (PubChem release ) Monoisotopic Mass:. - Here, Mn is in +3 oxidation state. Hexacyanides M(CN) 6 3− (M = Ti, V, Cr, Mn, Fe, Co), which are octahedral in shape.
Total Ni 2+ = Ni 2+ + NiL 4 2+ If the solution contains 1.6 x 10-4 % of the Ni in the free form then this means that:. The complex M n (C N) 6 4 − is Having magnetic moment 1.73 BM It is low spin complex, paraamagnetic ion and inner orbital complex. Determining oxidation numbers from the Lewis structure (Figure 1a) is even easier than deducing it from the molecular formula (Figure 1b).
Mn(CN)63-, Fe(CN)63-and Co(C2O4)33- are inner orbital complexes involving d2sp3 hybridisation,the former two complexes are paramagnetic and the latter diamagnetic. Ni ----- X 100 = 1.6 x 10 ^-4 (equation 5.1) Ni + NiL4 But since the amount of free Ni 2+ is extremely small then the Total Ni(II) can be approximated to NiL 4 2+. Square planar coordination can be imagined to result when two ligands on the z-axis of an octahedron are removed from the complex, leaving.
Supplementary tables, structure figures, some magnetic data, additional theoretical analysis of magnetic behavior. (c) Mn(CN) 6 4− or MnCl 6 4− Trimethylphosphine, P(CH 3 ) 3 , can act as a ligand by donating the lone pair of electrons on the phosphorus atom. (ii) Inner or outer orbital complex.
Central metal ion or atom + ligands + counter ion (if needed) • Called complex ion if charged. And the increase in coordination number, with fluoride ion, from 4 to 6 to 7 for B 3 +, Fe 3 +, and Zr 4 + is the result mainly of the increase in size of the. Sir/Madam can you please explain how this result was obtained.
The kinetics of the Mn(CN) 6 4− + e − ⇌ Mn(CN) 6 5− system at mercury electrodes has been investigated using cyclic voltammetry, chronocoulometric and faradaic impedance methods. Manganese ( mn ) Mn2+ Fe2+ Tin ( Sn ) vanadium N2 (2-) C2 2+ Strontium K ( Potassium ) na mg ( Magnesium ) o al3 ti2 cu2 Cl Titanium ( ti ) Diamagnetic List C2 Ne2 CO silicon ( si ) sulfur ( s ). Mn 3+ + e − ⇌ Mn 2+ 1.5.
The color of \(\ce{Fe(H2O)6^3+}\) is violet, and \(\ce{Fe(NH3)6^3+}\) is yellow in color. 6 CN ligands must provide a total charge of −6. What can you conclude about the effects of the different ligands on the magnitude of ??.
A record-breaking high-spin state is observed in a molecule based on the Mo(CN) 7 4− building block. Carries a charge of −1. As each ligand carries –1 charge, the oxidation number of Mn must be +2.
The crystal field is octahedral. Fe(CN) 6 4– has Fe in the +2 oxidation state, which is d 6, and a strong octahedral crystal field so LFSE = –24Dq + 2P. Atomic number of Mn, Fe, Co and Ni are 25, 26 27 and 28 respectively.
For example, it has been shown that in the d 1 hexacyano titanium complex, Ti(CN) 6 3-, the equatorial Ti-C bond lengths are 2.168 Å, whereas the axial bond lengths are 2.199 Å, a difference of about 13%. The size of the R groups changes the structure enough that it is locked into high-spin species at all temperatures. Chemistry MCQs for Class 12 Chapter Wise with Answers PDF Download was Prepared Based on Latest Exam Pattern.
Organization of Electrons in Atoms;. Water is a weak field ligand. Therefore, the arrangement of the electrons in Mn(H 2O) 6 2+is t 2g 3e g 2.
Mn(CN) 6 3- , Co(NH 3) 6 3+, Cr(H 2 O) 6 3+, FeCl 6 4-(i) Type of hybridisation. Computed by PubChem 2.1 (PubChem release ) Topological Polar Surface Area:. K 4 Mn (CN) 6 is a coordination compound.
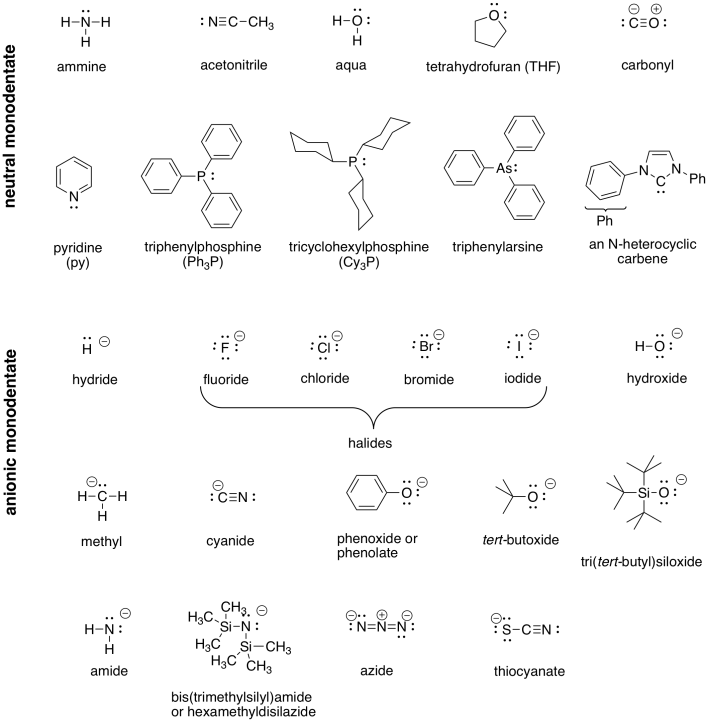
The forces that hold the atoms together in these complexes and that lead to the observed coordination numbers are of different kinds. Ligand charge transfer (MLCT) like in Fe(bpy) 3 2+. The cyanide anion is a ligand for many transition metals.
(a) For the complex Fe(CN) 6 3–, write the hybridization type, magnetic character and spin nature of the complex. Question_answer8) Explain Co(NH3)63+ is an inner orbital complex whereas is an outer orbital complex. When CN6 comes it needs 6 orbitals but the available orbitals are 5 only (one S, 1 orbital of d as rest are filled with singe electrons and 3 orbital.
24.1 The Structure of Complexes • Contain coordinate covalent bonds • Unusual composition:. It is usually available as the salt potassium ferrocyanide, which has the formula K 4 Fe(CN) 6. The complex Mn (H 2 O) 6 2+ has five unpaired electrons, whereas Mn (CN) 6 4− has only one.
Thus, we expect the compound to be low-spin. • Fe(CN)6 4. The bonds to the highly electronegative fluorine atoms in the fluoride complexes are essentially ionic;.
Share on Facebook Tweet on Twitter. Computed by Cactvs 3.4.6.11 (PubChem release ) Exact Mass:. 4-, the d-electron count on Mn(VII) is d 0.
Mn is element #25, so it has 25 protons and electrons.
Www Unf Edu Michael Lufaso Chem3610 Inorganic Chapter Pdf
21 3 Spectroscopic And Magnetic Properties Of Coordination Compounds General Chemistry 1 2

Coordination Chemistry

Mncn 6 4 On The Left And Na 4 Mncn 6 On The Right Orbitals Download Scientific Diagram

Ncert Exemplar Class 12 Chemistry Chapter 9 Coordination Compounds Learn Cbse
Coordination Chemistry

What Is The Hybridisation Of Mn Cn 6 3 Quora

Introduction To The Transition Elements Ppt Download
21 Complex Ions Coordination Compounds Chemistry Libretexts

Using Valence Bond Theory Explain The Following In Relation To The Complexes Given Below Mn Cn 6 3 Co Nh3 6 3 Cr Ho 6 3 Frcl6 4 I Type Of Hybridisation Ii Inner Or Outer

Bonding In Coordination Compounds Ppt Video Online Download

Ncert Exemplar Class 12 Chemistry Chapter 9 Coordination Compounds Learn Cbse

Bonding In Coordination Compounds Ppt Video Online Download
Nroer Gov In Media 4 6 1 Af81db255cef9fbf1fcac80beb7dda4cbd454e4 Pdf

Palladium Dicyanide Wikipedia

A Crystal Structure Of The Cationic Complex Mn 2 Bipym H 2 O 6 Download Scientific Diagram

Crystal Data And Structural Refinement Parameters For The Complexes 1 3 Download Table
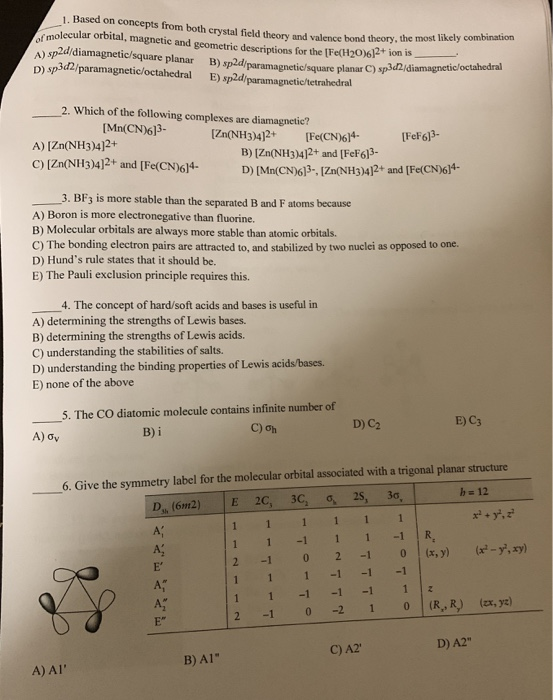
Solved 1 Based On Concepts From Both Crystal Field Theor Chegg Com
Nroer Gov In Media 4 6 1 Af81db255cef9fbf1fcac80beb7dda4cbd454e4 Pdf
What Is Fe S Oxidation Number In K3 Fe Cn 6 Quora
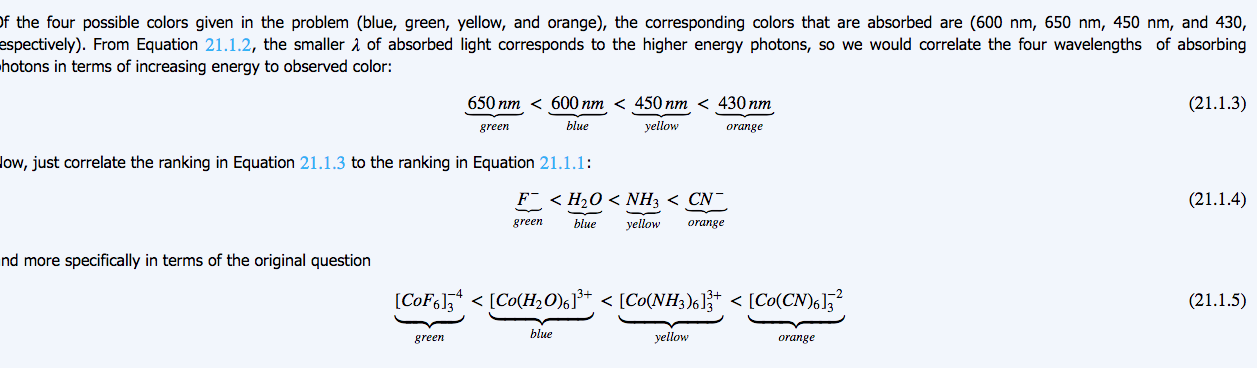
21 Complex Ions Coordination Compounds Chemistry Libretexts

What Is The Hybridization Of Cr Cn 6 4 Quora
What Is The Hybridization Of Fe Cn 6 3 Quora
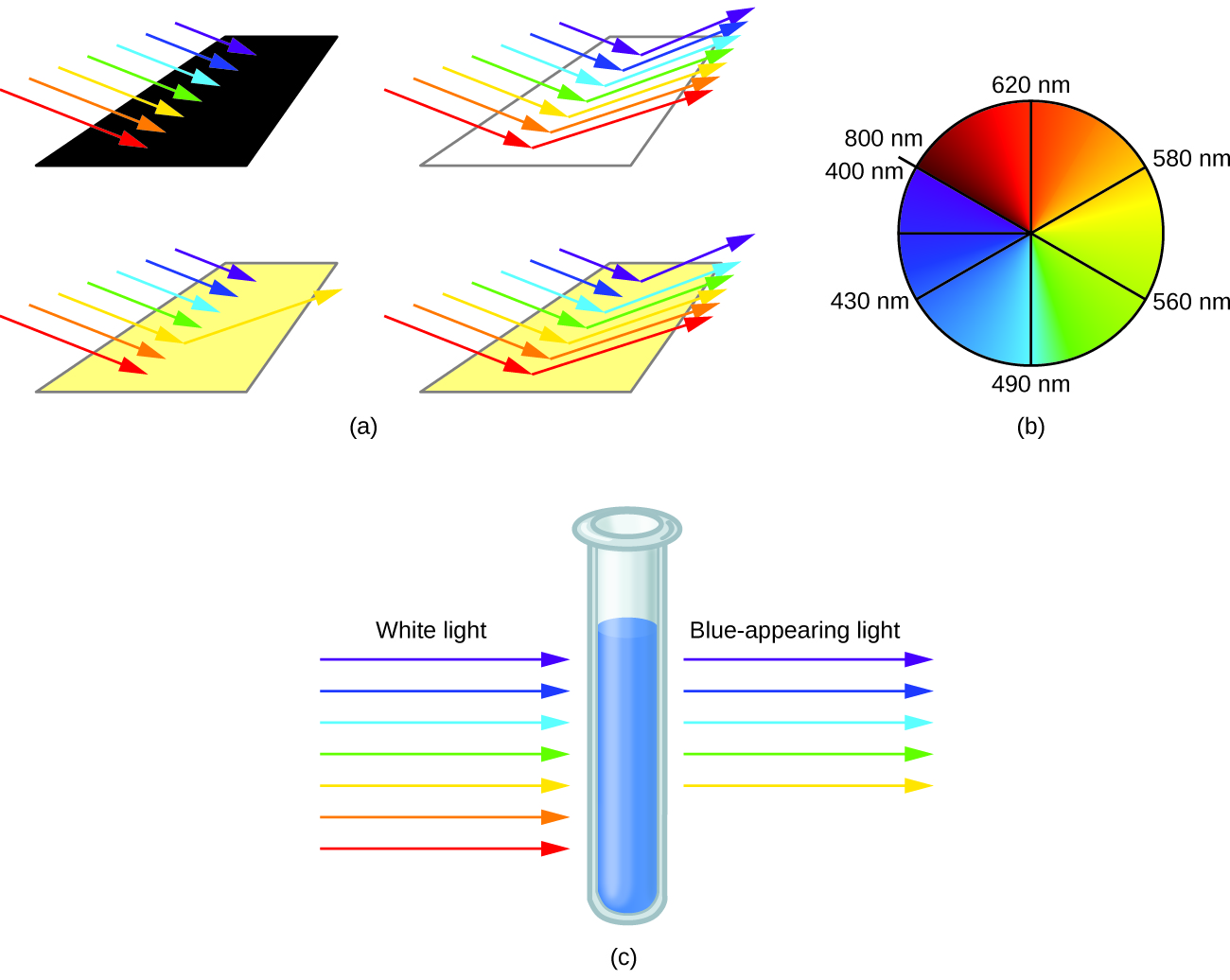
21 3 Spectroscopic And Magnetic Properties Of Coordination Compounds General Chemistry 1 2
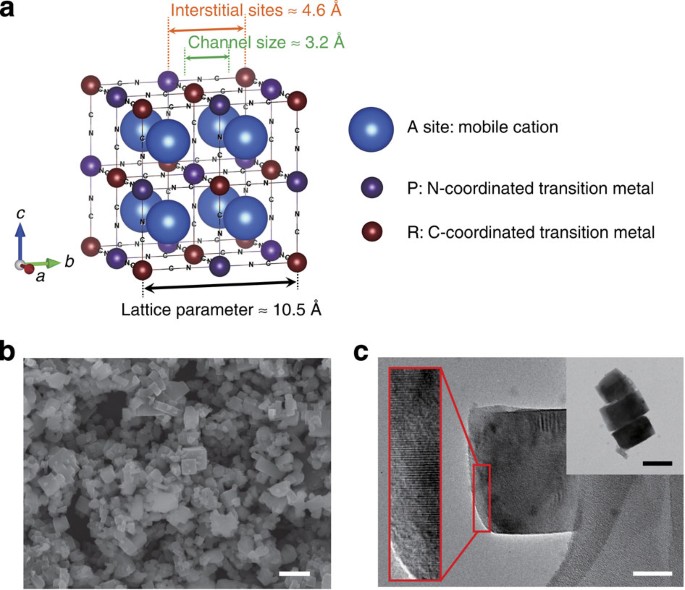
Manganese Hexacyanomanganate Open Framework As A High Capacity Positive Electrode Material For Sodium Ion Batteries Nature Communications
Nptel Ac In Content Storage2 Courses Questions answers coordination Pdf

Mncn 6 4 On The Left And Na 4 Mncn 6 On The Right Orbitals Download Scientific Diagram

Mncn 6 4 On The Left And Na 4 Mncn 6 On The Right Orbitals Download Scientific Diagram

Ncert Exemplar Class 12 Chemistry Chapter 9 Coordination Compounds Learn Cbse

Coordination Chemistry Ppt Download

A Crystal Structure Of Open Framework Na 2 Mn Mn Cn 6 61 B Download Scientific Diagram

Chapter 8 Worked Example 1 The Decavanadate Ion Is A Complex Species With Chemical Formula V 10 O It Reacts With An Excess Of Acid To Form The Dioxovanadium Ppt Download

What Is The Hybridization Of K4 Fe Cn 6 Quora

For The Complex Ion Fe Cn 6 3 State I The Type Of Hybridisation Ii The Magnetic Behaviour Iii The Oxidation Number Of The Central Metal Atom

Manganese Ii Chloride Wikipedia

Non Prussian Blue Structures And Magnetic Ordering Of Na2mn Ii Mn Ii Cn 6 And Na2mn Ii Mn Ii Cn 6 2h2o Semantic Scholar
Answer Key Chapter 19 Chemistry 2e Openstax
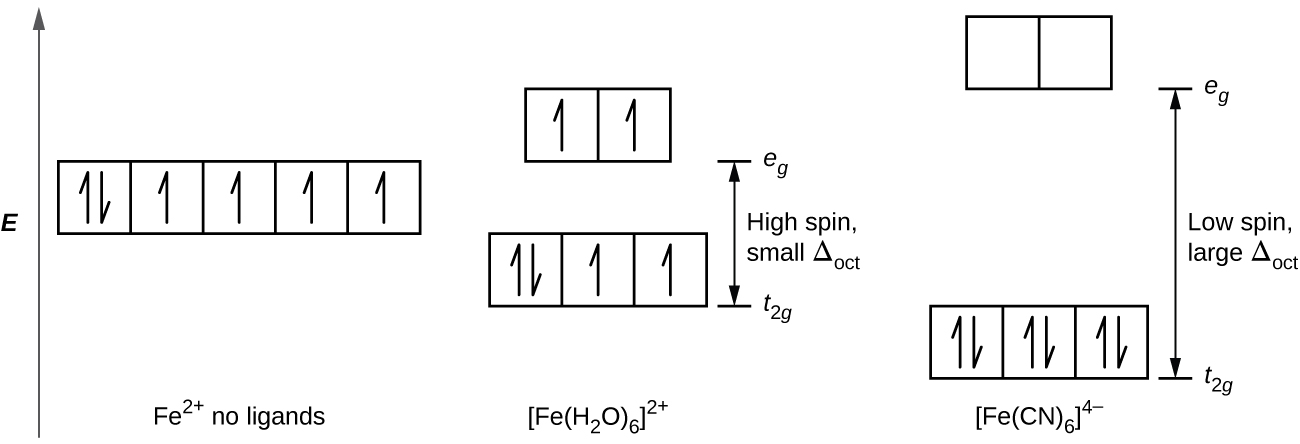
21 3 Spectroscopic And Magnetic Properties Of Coordination Compounds General Chemistry 1 2

Anomalous Stoichiometry And Antiferromagnetic Ordering For The Extended Hydroxymanganese Ii Cubes Hexacyanometalate Based 3d Structured Mnii4 Oh 4 Mnii Cn 6 Oh2 6 H2o Lapidus 19 Chemistry A European Journal Wiley Online Library
What Is The Hybridization Of Fe Cn 6 3 Quora

Solved 1 Name Four Factors Which Influence A Or At Spli Chegg Com

The Spin Only Magnetic Moments Of Mn Cn 6 4 Amp Mnbr4 2 In Bhor Magnetons Respectively Are Brainly In
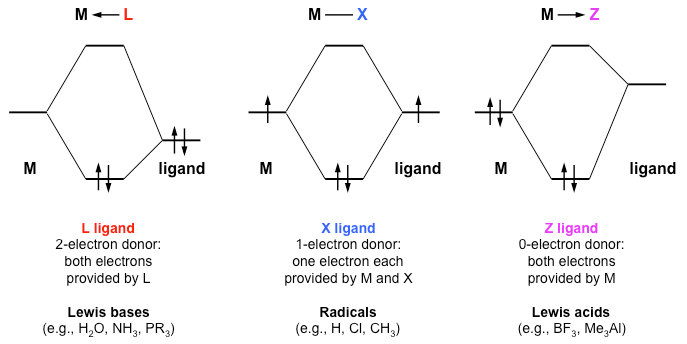
Introduction To Inorganic Chemistry Coordination Chemistry And Crystal Field Theory Wikibooks Open Books For An Open World
Nptel Ac In Content Storage2 Courses Questions answers coordination Pdf
What Is The Hybridization Of K3 Fe Cn 6 Quora
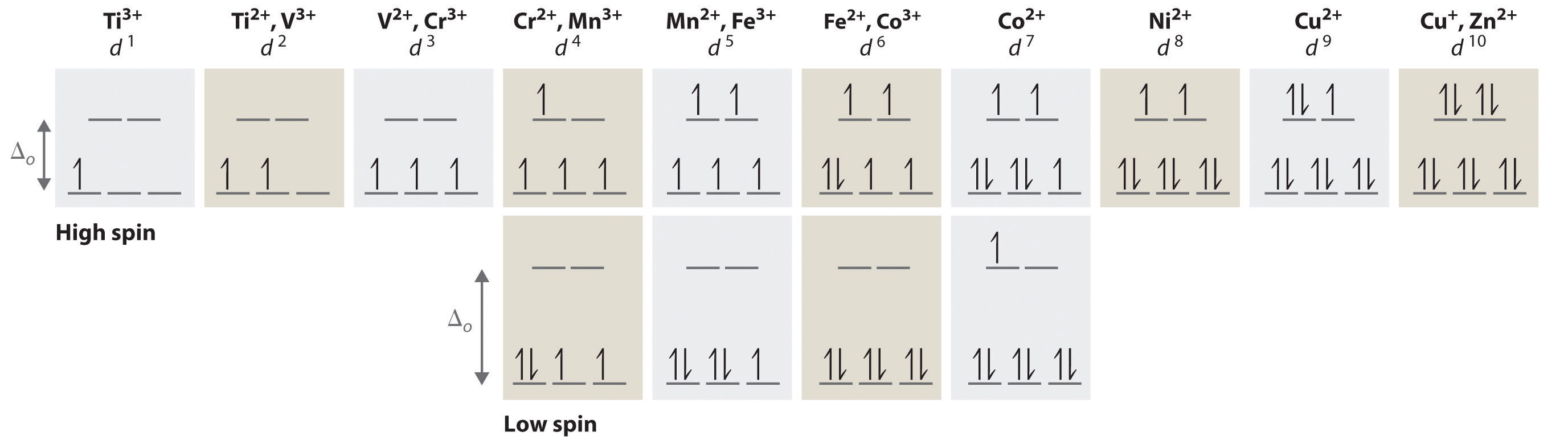
Introduction To Crystal Field Theory Chemistry Libretexts

Structure And Bonding In Low Spin Octahedral Manganese Ii Carbonyls Ligand Set Control Of Spin Delocalisation Pdf Document
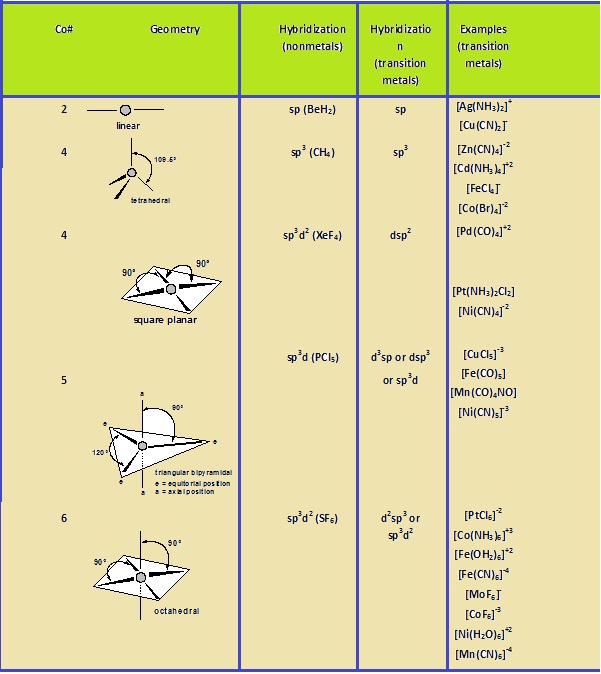
Chemistry World
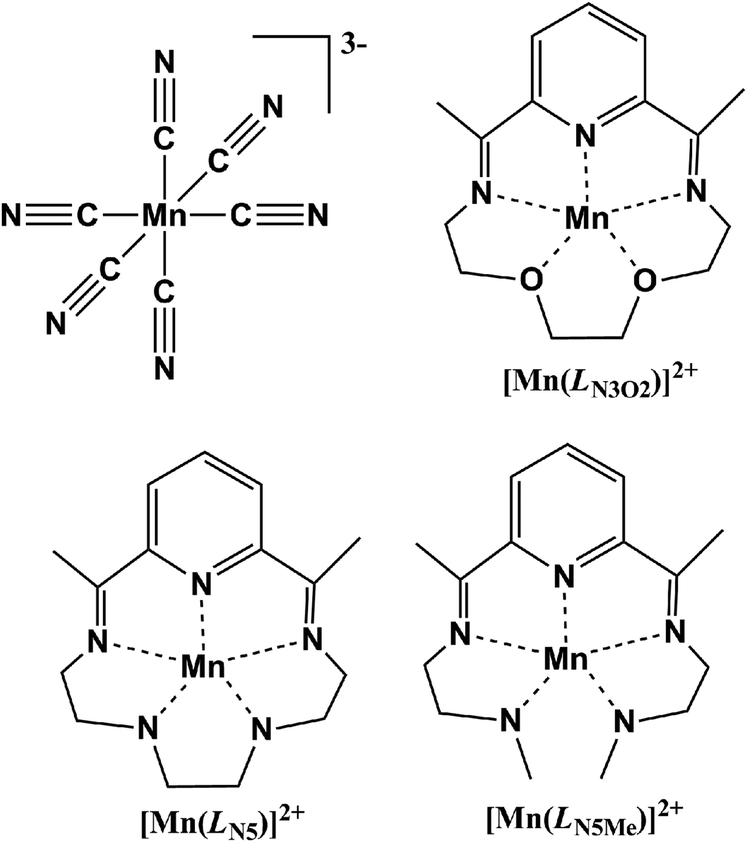
Syntheses Structures And Magnetic Properties Of Three New Cyano Bridged Complexes Based On The Mn Cn 6 3 Building Block Dalton Transactions Rsc Publishing Doi 10 1039 C5dtf

Solved Draw The Crystal Field Diagram For Mn Cn 6 4 A Chegg Com
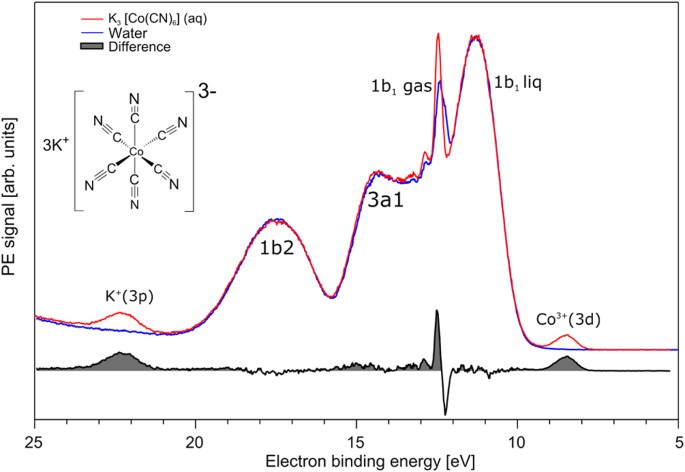
Chemical Bonding In Aqueous Hexacyano Cobaltate From Photon And Electron Detection Perspectives Scientific Reports
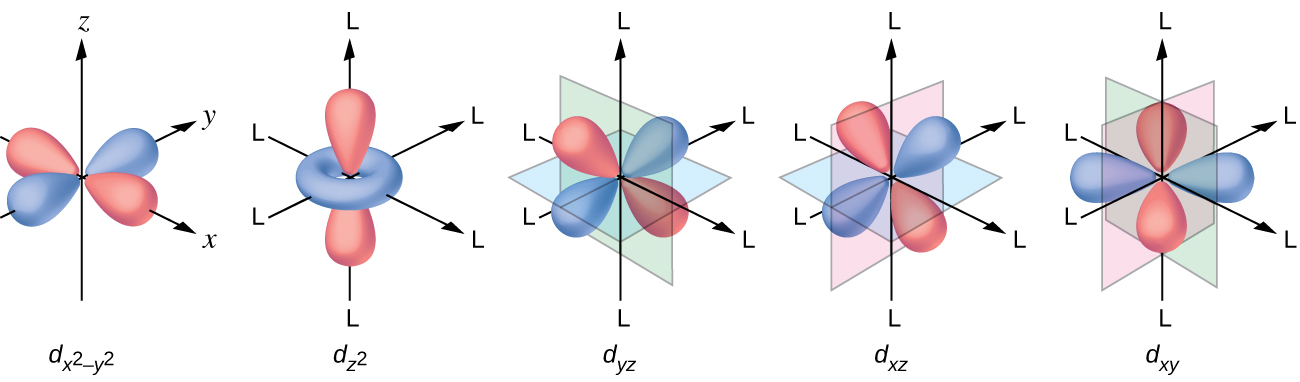
21 3 Spectroscopic And Magnetic Properties Of Coordination Compounds General Chemistry 1 2

Ncert Exemplar Class 12 Chemistry Chapter 9 Coordination Compounds Learn Cbse
Www Unf Edu Michael Lufaso Chem3610 Inorganic Chapter Pdf
Q Tbn 3aand9gcsfuc Fmyq6717czm2rssyuflbgb7viknkndv0h3dz0kleda28k Usqp Cau

Non Prussian Blue Structures And Magnetic Ordering Of Na2mn Ii Mn Ii Cn 6 And Na2mn Ii Mn Ii Cn 6 2h2o Semantic Scholar

What Is The Hybridisation Of Mn Cn 6 3 Quora
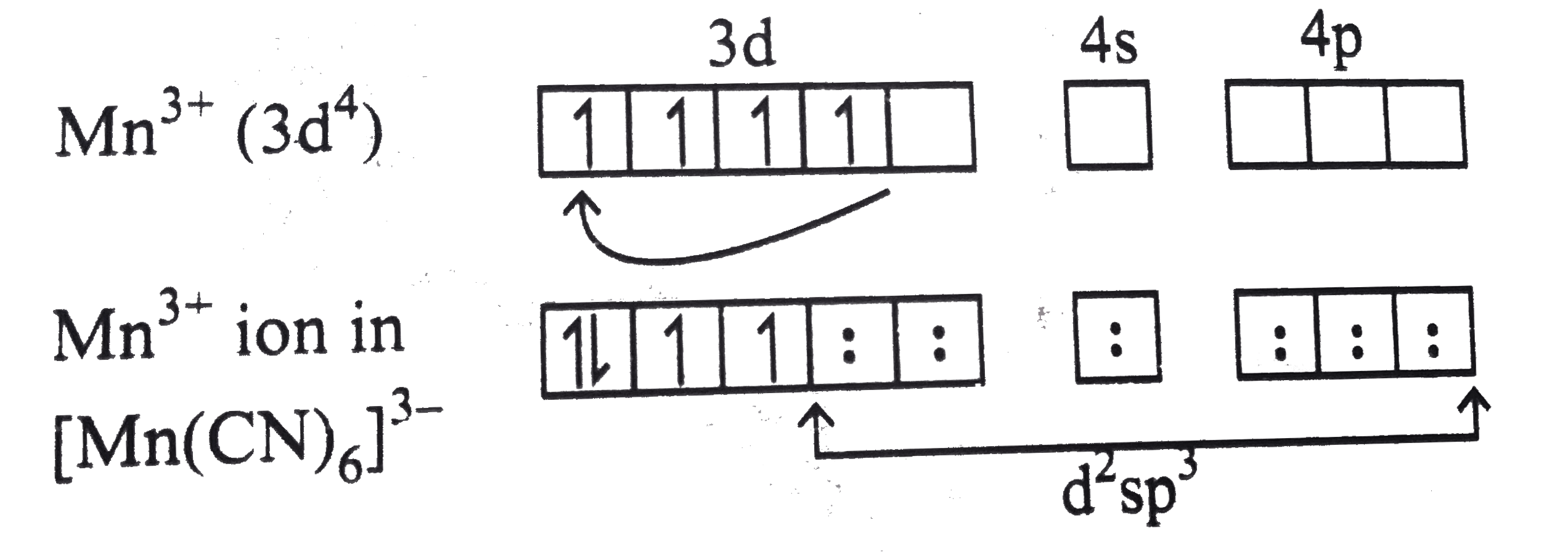
Magnetic Moment Value Of Mn Cn 6 3 Ion In 2 8bm Predic
How To Know That Nicl4 2 Has Tetrahedral Geometry Whereas Ni Cn 4 2 Has Square Planar Geometry Quora

Chapter 9 Coordination Compounds
The Ambient And High Pressure Structures Of A Mn Co Cn 6 0 67 And Download Scientific Diagram

Give The Number Of Unpaired Electrons In The Following Complex Ion With Reason I Fef 14 Ii Fe Cn 6

What Is The Oxidation Number Of Co Cn 6 3 Quora
Using Valence Bond Theory Explain The Following In Relation To The Complexes Given Below Sarthaks Econnect Largest Online Education Community
21 Complex Ions Coordination Compounds Chemistry Libretexts
Q Tbn 3aand9gct4xhrsnaxcn07lwqjubvsdbtmabeje321ap 6xv8k7ulqosfjd Usqp Cau

Structure Of Mn Cn 6 4 Mn Skuckxye Chemistry Electrochemistry Meritnation Com

Ni Co 4 Ni Cn 4 2 Ni Cl 4 2 Dsp2 Hybridization Structure Paramagnetic Diamagnetic Examples
Pick Out The Correct Statement With Respect To Mn Cn 6 3
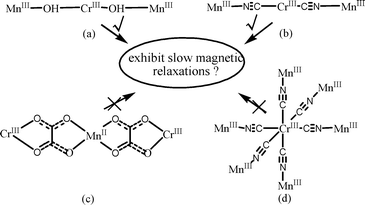
Synthesis Structure Magnetic Properties And Dft Calculations Of Two Hydroxo Bridged Complexes Based On Mniii Schiff Bases Dalton Transactions Rsc Publishing

How Is Ni Nh3 6 2 Paramagnetic While Ni Cn 6 4 Diamagnetic Quora

Bonding In Coordination Compounds Ppt Video Online Download
Http Ssc Maheshtutorials Com Images Chemistry Jeemain Chem Co Ordinationcompounds Exercise 3 Pdf

Important Questions For Cbse Class 12 Chemistry Coordination Compounds Cbse Tuts
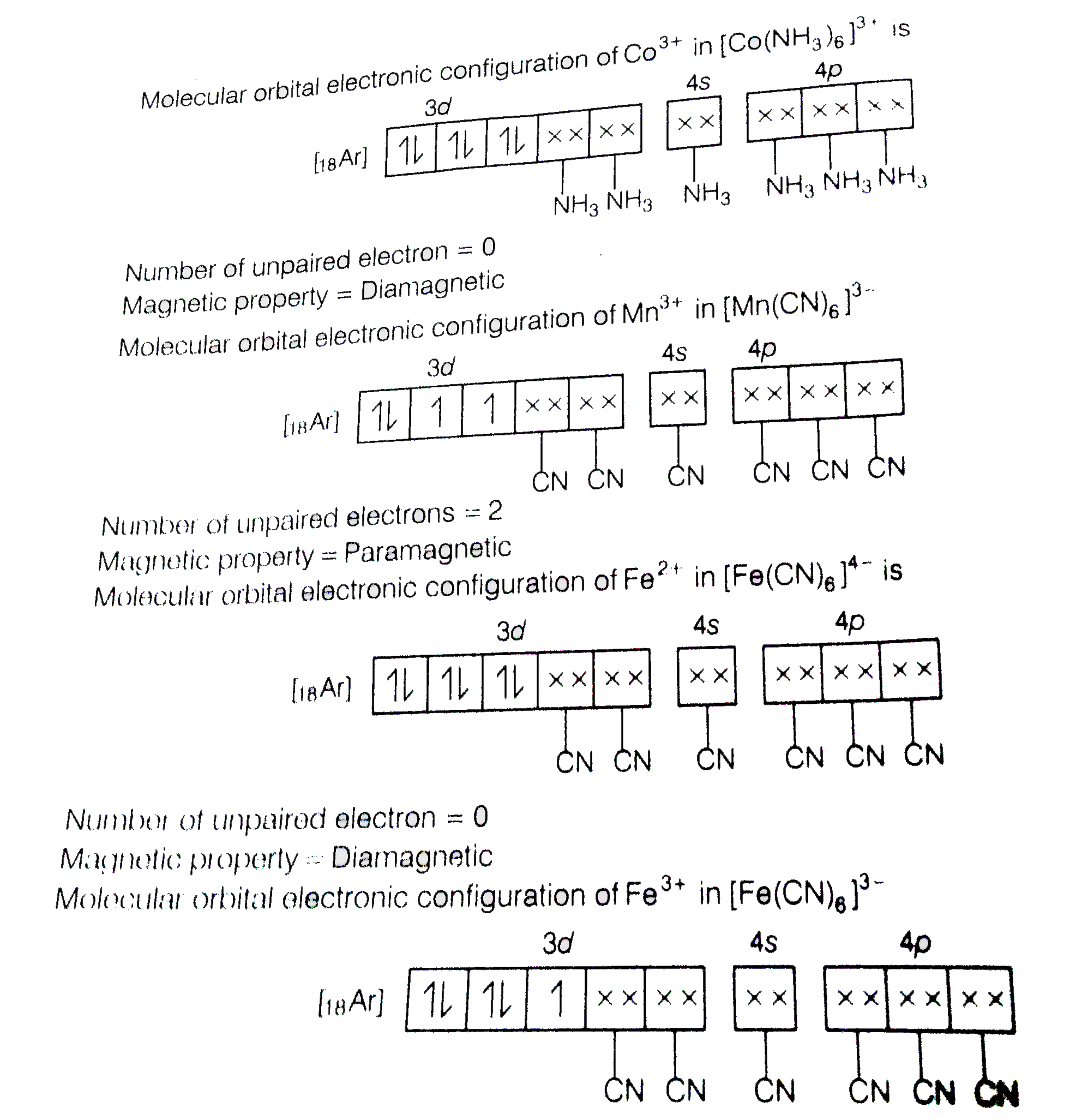
Atomic Number Of Mn Fe And Co Are 25 26 And 27 Respectively Whi

Mncn 6 4 On The Left And Na 4 Mncn 6 On The Right Orbitals Download Scientific Diagram
Sample Questions Chapter 25
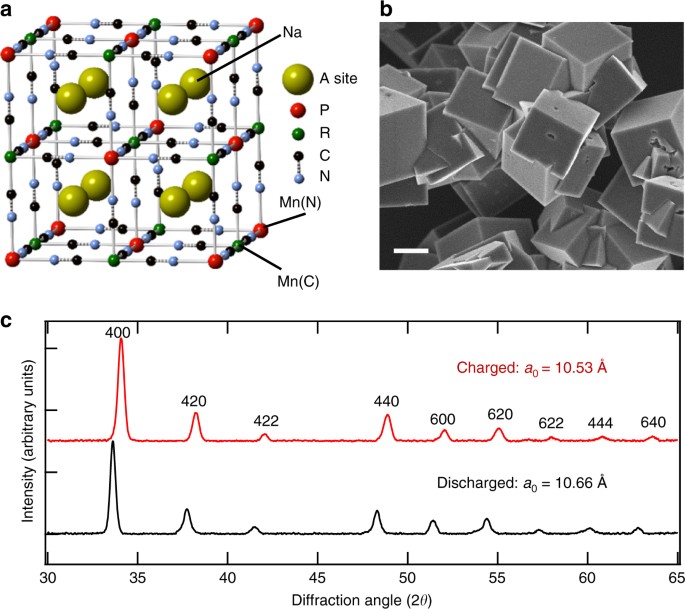
Monovalent Manganese Based Anodes And Co Solvent Electrolyte For Stable Low Cost High Rate Sodium Ion Batteries Nature Communications
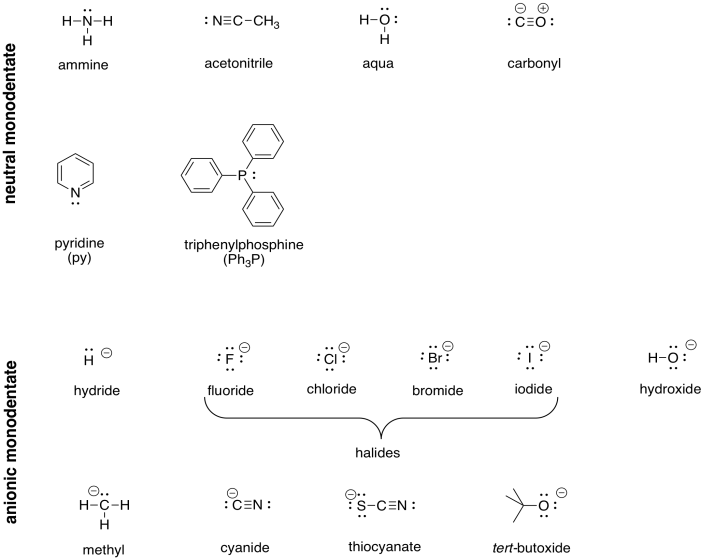
Transition Metal Complexes

25 Write The Oxidation State Coordinationnumber Nature Of Ligand Magnetic Propertyand Electronic Brainly In
How To Know That Nicl4 2 Has Tetrahedral Geometry Whereas Ni Cn 4 2 Has Square Planar Geometry Quora
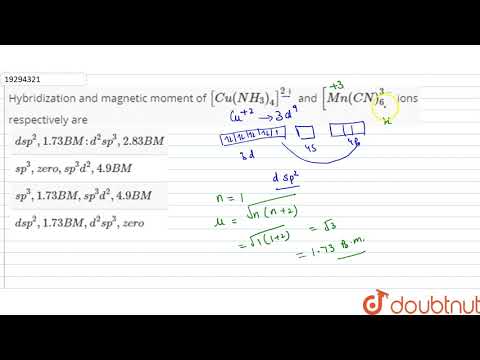
Youtube
Q Tbn 3aand9gcslz7w Irz42eipsy5ui9 2njn2 Mojggd Q8jzigpicqj O01l Usqp Cau

Magnetic Behaviour Of Complex Compounds Chemistry Stack Exchange

Iron Transition Metal Chemistry Iron Ii Fe2 Iron Iii Fe3 Complexes Ions Ligand Substitution Redox Chemical Reactions Principal Oxidation States 2 3 Extraction Gce As Ib A Level Inorganic Chemistry Revision Notes
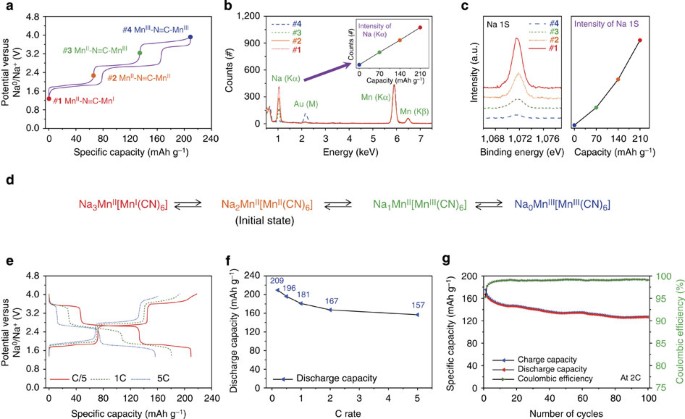
Manganese Hexacyanomanganate Open Framework As A High Capacity Positive Electrode Material For Sodium Ion Batteries Nature Communications
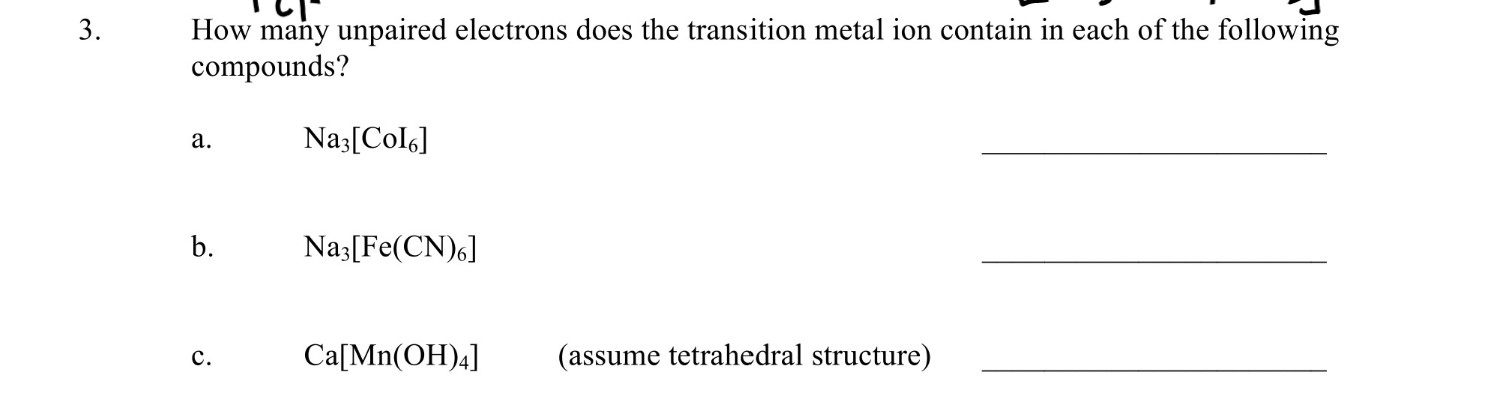
Solved 3 How Many Unpaired Electrons Does The Transition Chegg Com

How Is Cr Nh3 6 3 Paramagnetic And Co Nh3 6 3 Is Diamagnetic Quora
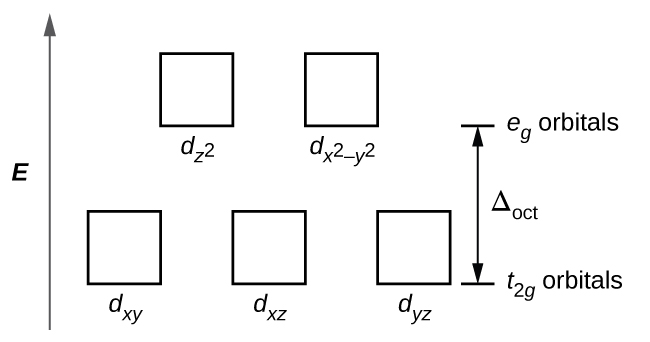
21 3 Spectroscopic And Magnetic Properties Of Coordination Compounds General Chemistry 1 2
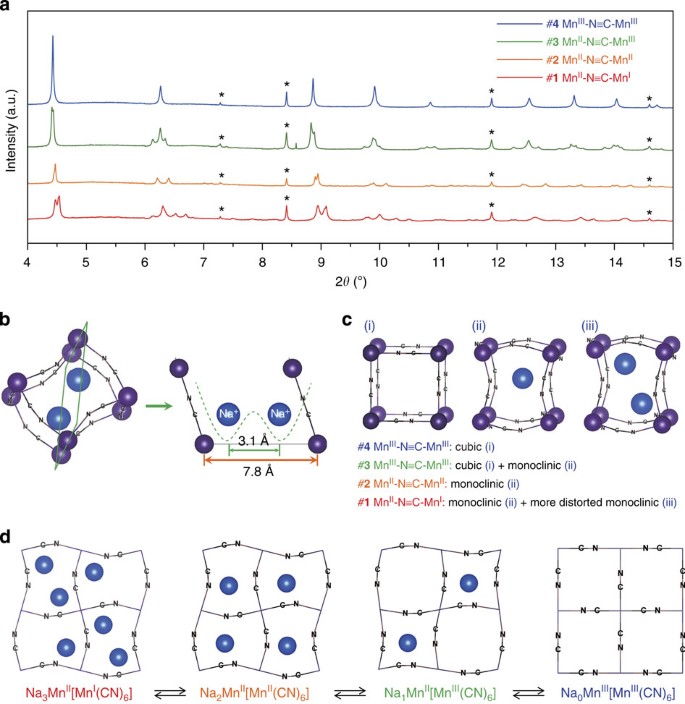
Manganese Hexacyanomanganate Open Framework As A High Capacity Positive Electrode Material For Sodium Ion Batteries Nature Communications
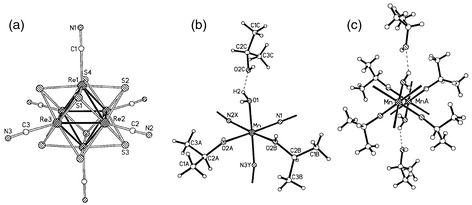
Extended Framework Materials Incorporating Cyanide Cluster Complexes Structure Of The First 3d Architecture Accommodating Organic Molecules Chemical Communications Rsc Publishing

Mncn 6 4 On The Left And Na 4 Mncn 6 On The Right Orbitals Download Scientific Diagram
Q Tbn 3aand9gctmg7cjydnldzluwdseeonpvnegvymrxom4cf5jhadbpcl6uvgc Usqp Cau
Www Unf Edu Michael Lufaso Chem3610 Inorganic Chapter Pdf

Introduction To Inorganic Chemistry Coordination Chemistry And Crystal Field Theory Wikibooks Open Books For An Open World

Magnetic Behaviour Of Complex Compounds Chemistry Stack Exchange

Pdf Anomalous Non Prussian Blue Structures And Magnetic Ordering Of K 2 Mn Ii Mn Ii Cn 6 And Rb 2 Mn Ii Mn Ii Cn 6 Semantic Scholar

Ncert Solutions For Class 12 Chemistry Chapter 9 Coordination Compounds Cbse Tuts



